当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Dimethyl 2,2′-Azobis(2-methylpropionate) in 15 Pure Solvents and in a Methanol + Water Binary Solvent System
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.jced.9b00842 Yang Li 1 , Yang Zhang 1 , Xue Zhong Wang 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.jced.9b00842 Yang Li 1 , Yang Zhang 1 , Xue Zhong Wang 1, 2
Affiliation
|
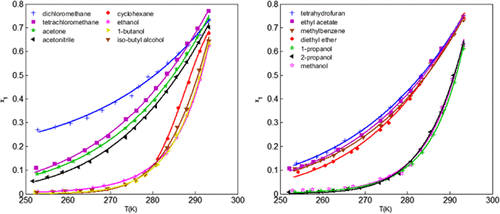
The solubility of dimethyl 2,2′-azobis(2-methylpropionate) (AIBME) was determined in 15 pure solvents, including cyclohexane, dichloromethane, diethyl ether, acetone, tetrahydrofuran, ethyl acetate, tetrachloromethane, acetonitrile, methylbenzene, methanol, ethanol, n-propyl alcohol, isopropanol, n-butyl alcohol, and iso-butyl alcohol, and in a binary solvent system (methanol + water) at the temperature range from 253.15 to 293.15 K at atmospheric pressure. Between 278.15 and 293.15 K, the solubility of AIBME is more sensitive to temperature in alcohol solvents than in other solvents. However, the solubility of AIBME in nonalcoholic solvents decreased more rapidly than in alcohol solvents below 278.15 K. The AIBME solubility in the binary solvent system (methanol + water) is positive to temperature and the initial mole fraction of methanol. The measured solubility data of AIBME in pure solvents were correlated by the modified Apelblat equation, the λh equation, the nonrandom two-liquid (NRTL) model, and the Wilson model. The measured solubility data of AIBME in binary solvent systems were correlated by the modified Apelblat equation, the nonrandom two-liquid (NRTL) model, and the Wilson model. Among the four models, the NRTL model is the best model to fit the solubility of AIBME, especially in alcohol solvents. In addition, the mixing properties of AIBME in 15 pure solvents, including standard mixing Gibbs energy, mixing enthalpy, and mixing entropy, were calculated and discussed. The results provide useful information for practical optimization of AIBME crystallization processes.
中文翻译:

2,2'-偶氮双(2-甲基丙酸酯)二甲基在15种纯溶剂和甲醇+水二元溶剂体系中的溶解度
在15种纯溶剂中测定了2,2'-偶氮二(2-甲基丙酸二甲酯)(AIBME)的溶解度,包括环己烷,二氯甲烷,乙醚,丙酮,四氢呋喃,乙酸乙酯,四氯甲烷,乙腈,甲基苯,甲醇,乙醇,ñ丙基醇,异丙醇,ñ丁醇和异丁醇,并在二元溶剂系统(甲醇+水)中,在大气压下,温度范围为253.15至293.15K。在278.15和293.15 K之间,AIBME在醇类溶剂中的溶解度比温度对其他溶剂的敏感性更高。但是,AIBME在非醇类溶剂中的溶解度下降速度低于在278.15 K以下的醇类溶剂。在二元溶剂系统(甲醇+水)中,AIBME溶解度对温度和甲醇的初始摩尔分数呈正值。在纯溶剂AIBME的测量的溶解度数据是由改性Apelblat方程,λ相关ħ方程,非随机两液(NRTL)模型和Wilson模型。通过修正的Apelblat方程,非随机两液(NRTL)模型和Wilson模型,将AIBME在二元溶剂系统中测得的溶解度数据进行关联。在这四个模型中,NRTL模型是最适合AIBME溶解度的模型,尤其是在酒精溶剂中。此外,还计算并讨论了AIBME在15种纯溶剂中的混合性能,包括标准混合吉布斯能量,混合焓和混合熵。结果为AIBME结晶过程的实际优化提供了有用的信息。
更新日期:2020-02-21
中文翻译:

2,2'-偶氮双(2-甲基丙酸酯)二甲基在15种纯溶剂和甲醇+水二元溶剂体系中的溶解度
在15种纯溶剂中测定了2,2'-偶氮二(2-甲基丙酸二甲酯)(AIBME)的溶解度,包括环己烷,二氯甲烷,乙醚,丙酮,四氢呋喃,乙酸乙酯,四氯甲烷,乙腈,甲基苯,甲醇,乙醇,ñ丙基醇,异丙醇,ñ丁醇和异丁醇,并在二元溶剂系统(甲醇+水)中,在大气压下,温度范围为253.15至293.15K。在278.15和293.15 K之间,AIBME在醇类溶剂中的溶解度比温度对其他溶剂的敏感性更高。但是,AIBME在非醇类溶剂中的溶解度下降速度低于在278.15 K以下的醇类溶剂。在二元溶剂系统(甲醇+水)中,AIBME溶解度对温度和甲醇的初始摩尔分数呈正值。在纯溶剂AIBME的测量的溶解度数据是由改性Apelblat方程,λ相关ħ方程,非随机两液(NRTL)模型和Wilson模型。通过修正的Apelblat方程,非随机两液(NRTL)模型和Wilson模型,将AIBME在二元溶剂系统中测得的溶解度数据进行关联。在这四个模型中,NRTL模型是最适合AIBME溶解度的模型,尤其是在酒精溶剂中。此外,还计算并讨论了AIBME在15种纯溶剂中的混合性能,包括标准混合吉布斯能量,混合焓和混合熵。结果为AIBME结晶过程的实际优化提供了有用的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号