当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biotransformation Mechanism of Pesticides by Cytochrome P450: A DFT Study on Dieldrin.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.chemrestox.0c00013 Lihong Chai 1 , Shujing Ji 1 , Shubin Zhang 2, 3 , Haiying Yu 4 , Meirong Zhao 5 , Li Ji 1, 3
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.chemrestox.0c00013 Lihong Chai 1 , Shujing Ji 1 , Shubin Zhang 2, 3 , Haiying Yu 4 , Meirong Zhao 5 , Li Ji 1, 3
Affiliation
|
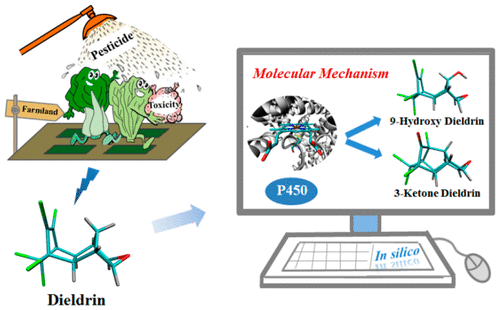
Pesticide biotransformation, especially by cytochrome P450 enzymes (CYPs), may produce metabolites with substantially altered toxicological and physicochemical profiles, which has drawn great attention as a basis for environmental risk assessment. CYPs are active in the metabolism of various reactions of pesticides, and there are potentially different short-lived oxidant species in CYPs (Compound I vs Compound 0), which make elucidating their biotransformation mechanism challenging. To facilitate this task, we performed density functional theory (DFT) calculations to explore the puzzling bifurcation pathways of dieldrin by CYPs. The results show that the two-oxidant mechanism does not work, while the bifurcation pathways are within the mechanistic framework of a two-state reactivity of Compound I. Specifically, 9-hydroxy-dieldrin as a hydroxylation product is formed via H-abstraction and essentially barrierless C-9 alkyl radical rebound in the doublet state; while 3-ketone-dieldrin as a dechlorination product is formed via H-abstraction, C-9 alkyl radical cyclization, and C-3 cyclized radical rebound in the quartet state followed by HCl elimination, originating from a significant barrier for C-9 alkyl radical rebound in the quartet state to provide this radical sufficient lifetime for cyclization. Thus, the ratio [dechlorination]/[hydroxylation] can be estimated as 1:35, consistent with the experimental findings. We envision that application of computational chemistry has a great potential in revealing the complex biotransformation mechanisms of pesticides.
中文翻译:

细胞色素P450对农药的生物转化机理:狄氏剂的DFT研究。
农药的生物转化,特别是通过细胞色素P450酶(CYPs)的生物转化,可能会产生具有明显改变的毒理学和理化特性的代谢产物,这已引起环境风险评估的高度重视。CYPs在农药的各种反应的代谢中具有活性,并且CYPs中可能存在不同的短期氧化剂(化合物I与化合物0),这使得阐明其生物转化机制具有挑战性。为了简化此任务,我们进行了密度泛函理论(DFT)计算,以探索CYP使狄氏剂产生令人费解的分叉途径。结果表明,两种氧化剂的机制不起作用,而分叉途径在化合物I的两种状态反应性的机制框架内。作为羟基化产物的9-羟基-狄氏剂通过H-提取和在双峰态下基本上无障碍的C-9烷基自由基反弹形成;而通过脱氢,C-9烷基自由基环化和C-3环化的自由基反弹成四重态并随后去除HCl形成了作为脱氯产物的3-酮-狄氏剂,这是由于C-9烷基的显着屏障引起的在四重态下进行自由基反弹,从而为环化反应提供足够的自由基寿命。因此,与实验结果一致,[脱氯] / [羟基化]之比可以估计为1:35。我们设想计算化学的应用在揭示农药复杂的生物转化机理方面具有巨大的潜力。
更新日期:2020-02-21
中文翻译:

细胞色素P450对农药的生物转化机理:狄氏剂的DFT研究。
农药的生物转化,特别是通过细胞色素P450酶(CYPs)的生物转化,可能会产生具有明显改变的毒理学和理化特性的代谢产物,这已引起环境风险评估的高度重视。CYPs在农药的各种反应的代谢中具有活性,并且CYPs中可能存在不同的短期氧化剂(化合物I与化合物0),这使得阐明其生物转化机制具有挑战性。为了简化此任务,我们进行了密度泛函理论(DFT)计算,以探索CYP使狄氏剂产生令人费解的分叉途径。结果表明,两种氧化剂的机制不起作用,而分叉途径在化合物I的两种状态反应性的机制框架内。作为羟基化产物的9-羟基-狄氏剂通过H-提取和在双峰态下基本上无障碍的C-9烷基自由基反弹形成;而通过脱氢,C-9烷基自由基环化和C-3环化的自由基反弹成四重态并随后去除HCl形成了作为脱氯产物的3-酮-狄氏剂,这是由于C-9烷基的显着屏障引起的在四重态下进行自由基反弹,从而为环化反应提供足够的自由基寿命。因此,与实验结果一致,[脱氯] / [羟基化]之比可以估计为1:35。我们设想计算化学的应用在揭示农药复杂的生物转化机理方面具有巨大的潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号