当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(Thia)calixarenephosphonic Acids as Potent Inhibitors of the Nucleic Acid Chaperone Activity of the HIV-1 Nucleocapsid Protein with a New Binding Mode and Multitarget Antiviral Activity.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-02-21 , DOI: 10.1021/acsinfecdis.9b00290 Nicolas Humbert 1 , Lesia Kovalenko 1, 2 , Francesco Saladini 3 , Alessia Giannini 3 , Manuel Pires 1 , Thomas Botzanowski 4 , Sergiy Cherenok 5 , Christian Boudier 1 , Kamal K Sharma 1 , Eleonore Real 1 , Olga A Zaporozhets 2 , Sarah Cianférani 4 , Carole Seguin-Devaux 6 , Federica Poggialini 7 , Maurizio Botta 7 , Maurizio Zazzi 3 , Vitaly I Kalchenko 5 , Mattia Mori 7 , Yves Mély 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-02-21 , DOI: 10.1021/acsinfecdis.9b00290 Nicolas Humbert 1 , Lesia Kovalenko 1, 2 , Francesco Saladini 3 , Alessia Giannini 3 , Manuel Pires 1 , Thomas Botzanowski 4 , Sergiy Cherenok 5 , Christian Boudier 1 , Kamal K Sharma 1 , Eleonore Real 1 , Olga A Zaporozhets 2 , Sarah Cianférani 4 , Carole Seguin-Devaux 6 , Federica Poggialini 7 , Maurizio Botta 7 , Maurizio Zazzi 3 , Vitaly I Kalchenko 5 , Mattia Mori 7 , Yves Mély 1
Affiliation
|
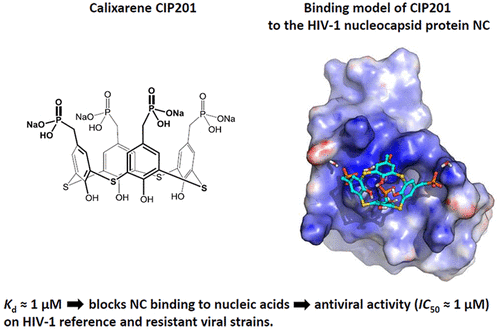
The nucleocapsid protein (NC) is a highly conserved protein that plays key roles in HIV-1 replication through its nucleic acid chaperone properties mediated by its two zinc fingers and basic residues. NC is a promising target for antiviral therapy, particularly to control viral strains resistant to currently available drugs. Since calixarenes with antiviral properties have been described, we explored the ability of calixarene hydroxymethylphosphonic or sulfonic acids to inhibit NC chaperone properties and exhibit antiviral activity. By using fluorescence-based assays, we selected four calixarenes inhibiting NC chaperone activity with submicromolar IC50 values. These compounds were further shown by mass spectrometry, isothermal titration calorimetry, and fluorescence anisotropy to bind NC with no zinc ejection and to compete with nucleic acids for the binding to NC. Molecular dynamic simulations further indicated that these compounds interact via their phosphonate or sulfonate groups with the basic surface of NC but not with the hydrophobic plateau at the top of the folded fingers. Cellular studies showed that the most soluble compound CIP201 inhibited the infectivity of wild-type and drug-resistant HIV-1 strains at low micromolar concentrations, primarily targeting the early steps of HIV-1 replication. Moreover, CIP201 was also found to inhibit the flipping and polymerization activity of reverse transcriptase. Calixarenes thus form a class of noncovalent NC inhibitors, endowed with a new binding mode and multitarget antiviral activity.
中文翻译:

(Thia)杯芳烃膦酸作为具有新的结合模式和多靶点抗病毒活性的HIV-1核壳蛋白的核酸伴侣活性的强抑制剂。
核衣壳蛋白(NC)是高度保守的蛋白,通过其两个锌指和碱性残基介导的核酸分子伴侣特性,在HIV-1复制中起关键作用。NC是抗病毒治疗的有希望的目标,特别是控制对当前可用药物具有抗药性的病毒株。由于已经描述了具有抗病毒特性的杯芳烃,我们探索了杯芳烃羟甲基膦酸或磺酸抑制NC分子伴侣性能并表现出抗病毒活性的能力。通过使用基于荧光的分析,我们选择了四种抑制NC伴侣活性的杯芳烃,其亚微摩尔IC50值较高。这些化合物进一步通过质谱,等温滴定热分析,荧光各向异性使NC结合而无锌射出,并与核酸竞争与NC的结合。分子动力学模拟进一步表明,这些化合物通过其膦酸酯或磺酸酯基与NC的基本表面相互作用,但与折叠手指顶部的疏水平台不相互作用。细胞研究表明,最可溶的化合物CIP201在低微摩尔浓度下抑制了野生型和耐药HIV-1菌株的感染性,主要针对HIV-1复制的早期步骤。此外,还发现CIP201抑制逆转录酶的翻转和聚合活性。杯芳烃因此形成一类非共价NC抑制剂,具有新的结合模式和多靶点抗病毒活性。分子动力学模拟进一步表明,这些化合物通过其膦酸酯或磺酸酯基与NC的基本表面相互作用,但与折叠手指顶部的疏水平台不相互作用。细胞研究表明,最可溶的化合物CIP201在低微摩尔浓度下抑制了野生型和耐药HIV-1菌株的感染性,主要针对HIV-1复制的早期步骤。此外,还发现CIP201抑制逆转录酶的翻转和聚合活性。杯芳烃因此形成一类非共价NC抑制剂,具有新的结合模式和多靶点抗病毒活性。分子动力学模拟进一步表明,这些化合物通过其膦酸酯或磺酸酯基与NC的基本表面相互作用,但与折叠手指顶部的疏水平台不相互作用。细胞研究表明,最可溶的化合物CIP201在低微摩尔浓度下抑制了野生型和耐药HIV-1菌株的感染性,主要针对HIV-1复制的早期步骤。此外,还发现CIP201抑制逆转录酶的翻转和聚合活性。杯芳烃因此形成一类非共价NC抑制剂,具有新的结合模式和多靶点抗病毒活性。
更新日期:2020-02-11
中文翻译:

(Thia)杯芳烃膦酸作为具有新的结合模式和多靶点抗病毒活性的HIV-1核壳蛋白的核酸伴侣活性的强抑制剂。
核衣壳蛋白(NC)是高度保守的蛋白,通过其两个锌指和碱性残基介导的核酸分子伴侣特性,在HIV-1复制中起关键作用。NC是抗病毒治疗的有希望的目标,特别是控制对当前可用药物具有抗药性的病毒株。由于已经描述了具有抗病毒特性的杯芳烃,我们探索了杯芳烃羟甲基膦酸或磺酸抑制NC分子伴侣性能并表现出抗病毒活性的能力。通过使用基于荧光的分析,我们选择了四种抑制NC伴侣活性的杯芳烃,其亚微摩尔IC50值较高。这些化合物进一步通过质谱,等温滴定热分析,荧光各向异性使NC结合而无锌射出,并与核酸竞争与NC的结合。分子动力学模拟进一步表明,这些化合物通过其膦酸酯或磺酸酯基与NC的基本表面相互作用,但与折叠手指顶部的疏水平台不相互作用。细胞研究表明,最可溶的化合物CIP201在低微摩尔浓度下抑制了野生型和耐药HIV-1菌株的感染性,主要针对HIV-1复制的早期步骤。此外,还发现CIP201抑制逆转录酶的翻转和聚合活性。杯芳烃因此形成一类非共价NC抑制剂,具有新的结合模式和多靶点抗病毒活性。分子动力学模拟进一步表明,这些化合物通过其膦酸酯或磺酸酯基与NC的基本表面相互作用,但与折叠手指顶部的疏水平台不相互作用。细胞研究表明,最可溶的化合物CIP201在低微摩尔浓度下抑制了野生型和耐药HIV-1菌株的感染性,主要针对HIV-1复制的早期步骤。此外,还发现CIP201抑制逆转录酶的翻转和聚合活性。杯芳烃因此形成一类非共价NC抑制剂,具有新的结合模式和多靶点抗病毒活性。分子动力学模拟进一步表明,这些化合物通过其膦酸酯或磺酸酯基与NC的基本表面相互作用,但与折叠手指顶部的疏水平台不相互作用。细胞研究表明,最可溶的化合物CIP201在低微摩尔浓度下抑制了野生型和耐药HIV-1菌株的感染性,主要针对HIV-1复制的早期步骤。此外,还发现CIP201抑制逆转录酶的翻转和聚合活性。杯芳烃因此形成一类非共价NC抑制剂,具有新的结合模式和多靶点抗病毒活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号