Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
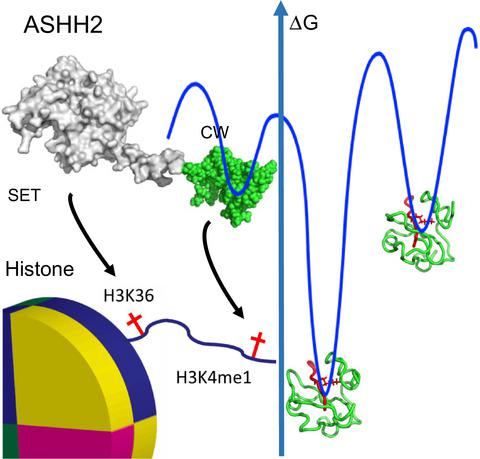
The Arabidopsis (ASHH2) CW domain binds monomethylated K4 of the histone H3 tail through conformational selection.
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-02-21 , DOI: 10.1111/febs.15256 Olena Dobrovolska 1 , Maxim Brilkov 1 , Noelly Madeleine 1, 2 , Øyvind Ødegård-Fougner 3 , Øyvind Strømland 2 , Stephen R Martin 4 , Valeria De Marco 5 , Evangelos Christodoulou 4 , Knut Teigen 2 , Johan Isaksson 6 , Jarl Underhaug 7 , Nathalie Reuter 7 , Reidunn B Aalen 8 , Rein Aasland 8 , Øyvind Halskau 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-02-21 , DOI: 10.1111/febs.15256 Olena Dobrovolska 1 , Maxim Brilkov 1 , Noelly Madeleine 1, 2 , Øyvind Ødegård-Fougner 3 , Øyvind Strømland 2 , Stephen R Martin 4 , Valeria De Marco 5 , Evangelos Christodoulou 4 , Knut Teigen 2 , Johan Isaksson 6 , Jarl Underhaug 7 , Nathalie Reuter 7 , Reidunn B Aalen 8 , Rein Aasland 8 , Øyvind Halskau 1
Affiliation
|
Chromatin post‐translational modifications are thought to be important for epigenetic effects on gene expression. Methylation of histone N‐terminal tail lysine residues constitutes one of many such modifications, executed by families of histone lysine methyltransferase (HKMTase). One such protein is ASHH2 from the flowering plant Arabidopsis thaliana, equipped with the interaction domain, CW, and the HKMTase domain, SET. The CW domain of ASHH2 is a selective binder of monomethylation at lysine 4 on histone H3 (H3K4me1) and likely helps the enzyme dock correctly onto chromatin sites. The study of CW and related interaction domains has so far been emphasizing lock–key models, missing important aspects of histone‐tail CW interactions. We here present an analysis of the ASHH2 CW‐H3K4me1 complex using NMR and molecular dynamics, as well as mutation and affinity studies of flexible coils. β‐augmentation and rearrangement of coils coincide with changes in the flexibility of the complex, in particular the η1, η3 and C‐terminal coils, but also in the β1 and β2 strands and the C‐terminal part of the ligand. Furthermore, we show that mutating residues with outlier dynamic behaviour affect the complex binding affinity despite these not being in direct contact with the ligand. Overall, the binding process is consistent with conformational selection. We propose that this binding mechanism presents an advantage when searching for the correct post‐translational modification state among the highly modified and flexible histone tails, and also that the binding shifts the catalytic SET domain towards the nucleosome.
中文翻译:

拟南芥(ASHH2)CW域通过构象选择结合组蛋白H3尾巴的单甲基化K4。
染色质的翻译后修饰被认为对于基因表达的表观遗传学作用很重要。组蛋白N末端赖氨酸残基的甲基化构成许多此类修饰之一,由组蛋白赖氨酸甲基转移酶(HKMTase)家族进行。一种这样的蛋白质是来自开花植物拟南芥的ASHH2。,其中包含交互域CW和HKMTase域SET。ASHH2的CW域是组蛋白H3(H3K4me1)上赖氨酸4处单甲基化的选择性结合物,可能有助于酶正确地停靠在染色质位点上。迄今为止,对CW和相关交互域的研究一直在强调锁定键模型,而缺少组蛋白尾部CW交互的重要方面。我们在这里使用NMR和分子动力学以及柔性线圈的突变和亲和力研究对ASHH2 CW-H3K4me1复合物进行分析。线圈的β增强和重排与复合物柔韧性的变化相吻合,特别是η1,η3和C末端的线圈,但在β1和β2链以及配体的C末端部分也是如此。此外,我们显示出具有异常动态行为的突变残基影响复杂的结合亲和力,尽管这些残基未与配体直接接触。总的来说,结合过程与构象选择是一致的。我们提出,当在高度修饰的和灵活的组蛋白尾部中寻找正确的翻译后修饰状态时,这种结合机制将显示出一个优势,并且该结合还将催化SET域移向核小体。
更新日期:2020-02-21
中文翻译:

拟南芥(ASHH2)CW域通过构象选择结合组蛋白H3尾巴的单甲基化K4。
染色质的翻译后修饰被认为对于基因表达的表观遗传学作用很重要。组蛋白N末端赖氨酸残基的甲基化构成许多此类修饰之一,由组蛋白赖氨酸甲基转移酶(HKMTase)家族进行。一种这样的蛋白质是来自开花植物拟南芥的ASHH2。,其中包含交互域CW和HKMTase域SET。ASHH2的CW域是组蛋白H3(H3K4me1)上赖氨酸4处单甲基化的选择性结合物,可能有助于酶正确地停靠在染色质位点上。迄今为止,对CW和相关交互域的研究一直在强调锁定键模型,而缺少组蛋白尾部CW交互的重要方面。我们在这里使用NMR和分子动力学以及柔性线圈的突变和亲和力研究对ASHH2 CW-H3K4me1复合物进行分析。线圈的β增强和重排与复合物柔韧性的变化相吻合,特别是η1,η3和C末端的线圈,但在β1和β2链以及配体的C末端部分也是如此。此外,我们显示出具有异常动态行为的突变残基影响复杂的结合亲和力,尽管这些残基未与配体直接接触。总的来说,结合过程与构象选择是一致的。我们提出,当在高度修饰的和灵活的组蛋白尾部中寻找正确的翻译后修饰状态时,这种结合机制将显示出一个优势,并且该结合还将催化SET域移向核小体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号