当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cell immaturity and white/beige adipocyte-potential of primary human adipose-derived stromal cells are restrained by culture-medium TGFβ1
STEM CELLS ( IF 4.0 ) Pub Date : 2020-02-26 , DOI: 10.1002/stem.3164 Hélène Leménager 1 , Loïc M A Fiévet 1 , Fabien Guilloton 1 , Abderrahim Naji 2 , Jean-Gérard Descamps 1 , Benoît Chaput 3 , Narufumi Suganuma 2 , Jean-Christophe Pagès 1 , Luc Sensebé 1 , Audrey Carrière 1 , Louis Casteilla 1 , Frédéric Deschaseaux 1
STEM CELLS ( IF 4.0 ) Pub Date : 2020-02-26 , DOI: 10.1002/stem.3164 Hélène Leménager 1 , Loïc M A Fiévet 1 , Fabien Guilloton 1 , Abderrahim Naji 2 , Jean-Gérard Descamps 1 , Benoît Chaput 3 , Narufumi Suganuma 2 , Jean-Christophe Pagès 1 , Luc Sensebé 1 , Audrey Carrière 1 , Louis Casteilla 1 , Frédéric Deschaseaux 1
Affiliation
|
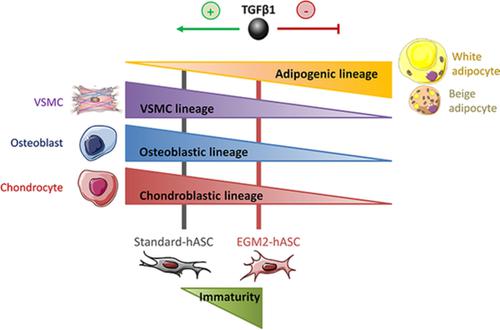
Human adipose‐derived stem/stromal cells (hASCs) can differentiate into specialized cell types and thereby contribute to tissue regeneration. As such, hASCs have drawn increasing attention in cell therapy and regenerative medicine, not to mention the ease to isolate them from donors. Culture conditions are critical for expanding hASCs while maintaining optimal therapeutic capabilities. Here, we identified a role for transforming growth factor β1 (TGFβ1) in culture medium in influencing the fate of hASCs during in vitro cell expansion. Human ASCs obtained after expansion in standard culture medium (Standard‐hASCs) and in endothelial cell growth medium 2 (EGM2‐hASCs) were characterized by high‐throughput transcriptional studies, gene set enrichment analysis and functional properties. EGM2‐hASCs exhibited enhanced multipotency capabilities and an immature phenotype compared with Standard‐hASCs. Moreover, the adipogenic potential of EGM2‐hASCs was enhanced, including toward beige adipogenesis, compared with Standard‐hASCs. In these conditions, TGFβ1 acts as a critical factor affecting the immaturity and multipotency of Standard‐hASCs, as suggested by small mother of decapentaplegic homolog 3 (SMAD3) nuclear localization and phosphorylation in Standard‐hASCs vs EGM2‐hASCs. Finally, the typical priming of Standard‐hASCs into osteoblast, chondroblast, and vascular smooth muscle cell (VSMC) lineages was counteracted by pharmacological inhibition of the TGFβ1 receptor, which allowed retention of SMAD3 into the cytoplasm and a decrease in expression of osteoblast and VSMC lineage markers. Overall, the TGFβ1 pathway appears critical in influencing the commitment of hASCs toward osteoblast, chondroblast, and VSMC lineages, thus reducing their adipogenic potential. These effects can be counteracted by using EGM2 culture medium or chemical inhibition of the TGFβ1 pathway.
中文翻译:

培养基 TGFβ1 抑制原代人脂肪来源基质细胞的细胞不成熟和白色/米色脂肪细胞潜能
人类脂肪来源的干/基质细胞 (hASC) 可以分化成特化的细胞类型,从而有助于组织再生。因此,hASCs 在细胞治疗和再生医学中引起了越来越多的关注,更不用说将它们从供体中分离出来的容易程度。培养条件对于扩大 hASC 同时保持最佳治疗能力至关重要。在这里,我们确定了培养基中转化生长因子 β1 (TGFβ1) 在影响体外细胞扩增过程中 hASC 命运方面的作用。在标准培养基 (Standard-hASCs) 和内皮细胞生长培养基 2 (EGM2-hASCs) 中扩增后获得的人类 ASCs 通过高通量转录研究、基因集富集分析和功能特性进行表征。与标准-hASCs 相比,EGM2-hASCs 表现出增强的多能性和不成熟的表型。此外,与标准 hASCs 相比,EGM2-hASCs 的脂肪生成潜力得到增强,包括朝向米色脂肪生成。在这些条件下,TGFβ1 作为影响标准 hASCs 的不成熟和多能性的关键因素,正如标准 hASCs 与 EGM2-hASCs 中十五位麻痹同系物 3 (SMAD3) 核定位和磷酸化的小母所暗示的那样。最后,标准 hASCs 进入成骨细胞、成软骨细胞和血管平滑肌细胞 (VSMC) 谱系的典型启动被 TGFβ1 受体的药理学抑制所抵消,这使得 SMAD3 保留在细胞质中并降低成骨细胞和 VSMC 的表达血统标记。全面的,TGFβ1 通路在影响 hASCs 对成骨细胞、软骨细胞和 VSMC 谱系的承诺方面似乎至关重要,从而降低了它们的成脂潜力。这些影响可以通过使用 EGM2 培养基或化学抑制 TGFβ1 途径来抵消。
更新日期:2020-02-26
中文翻译:

培养基 TGFβ1 抑制原代人脂肪来源基质细胞的细胞不成熟和白色/米色脂肪细胞潜能
人类脂肪来源的干/基质细胞 (hASC) 可以分化成特化的细胞类型,从而有助于组织再生。因此,hASCs 在细胞治疗和再生医学中引起了越来越多的关注,更不用说将它们从供体中分离出来的容易程度。培养条件对于扩大 hASC 同时保持最佳治疗能力至关重要。在这里,我们确定了培养基中转化生长因子 β1 (TGFβ1) 在影响体外细胞扩增过程中 hASC 命运方面的作用。在标准培养基 (Standard-hASCs) 和内皮细胞生长培养基 2 (EGM2-hASCs) 中扩增后获得的人类 ASCs 通过高通量转录研究、基因集富集分析和功能特性进行表征。与标准-hASCs 相比,EGM2-hASCs 表现出增强的多能性和不成熟的表型。此外,与标准 hASCs 相比,EGM2-hASCs 的脂肪生成潜力得到增强,包括朝向米色脂肪生成。在这些条件下,TGFβ1 作为影响标准 hASCs 的不成熟和多能性的关键因素,正如标准 hASCs 与 EGM2-hASCs 中十五位麻痹同系物 3 (SMAD3) 核定位和磷酸化的小母所暗示的那样。最后,标准 hASCs 进入成骨细胞、成软骨细胞和血管平滑肌细胞 (VSMC) 谱系的典型启动被 TGFβ1 受体的药理学抑制所抵消,这使得 SMAD3 保留在细胞质中并降低成骨细胞和 VSMC 的表达血统标记。全面的,TGFβ1 通路在影响 hASCs 对成骨细胞、软骨细胞和 VSMC 谱系的承诺方面似乎至关重要,从而降低了它们的成脂潜力。这些影响可以通过使用 EGM2 培养基或化学抑制 TGFβ1 途径来抵消。











































 京公网安备 11010802027423号
京公网安备 11010802027423号