当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient access to fluorescent benzofuro[3,2-b]carbazoles via TFA-promoted cascade annulations of sulfur ylides, 2-hydroxy-β-nitrostyrenes and indoles
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/21 , DOI: 10.1039/d0qo00137f Wenteng Chen 1, 2, 3, 4 , Feng Luo 1, 2, 3, 4 , You Wu 1, 2, 3, 4 , Jie Cen 1, 2, 3, 4 , Jiaan Shao 3, 4, 5, 6 , Yongping Yu 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020/02/21 , DOI: 10.1039/d0qo00137f Wenteng Chen 1, 2, 3, 4 , Feng Luo 1, 2, 3, 4 , You Wu 1, 2, 3, 4 , Jie Cen 1, 2, 3, 4 , Jiaan Shao 3, 4, 5, 6 , Yongping Yu 1, 2, 3, 4
Affiliation
|
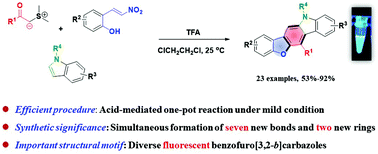
An efficient, TFA-promoted cascade annulation of sulfur ylides, 2-hydroxy-β-nitrostyrenes and indoles was established. This reaction results in the construction of a diverse set of fluorescent benzofuro[3,2-b]carbazoles with the generation of seven new bonds and two new rings in a one-pot transformation. A plausible mechanism for this reaction was proposed. Moreover, the photo-physical properties of the benzofuro[3,2-b]carbazoles were observed to be simply tuned by changing the electronic nature of the peripheral substituents in the core.
中文翻译:

通过TFA促进的硫代内酯,2-羟基-β-硝基苯乙烯和吲哚的级联反应有效获得荧光苯并呋喃[3,2-b]咔唑
建立了TFA促进的高效硫磺化物,2-羟基-β-硝基苯乙烯和吲哚的级联环化反应。该反应导致构建了一组不同的荧光苯并呋喃[3,2- b ]咔唑类化合物,并在一锅转化中生成了七个新键和两个新环。提出了该反应的合理机制。此外,观察到苯并呋喃并[3,2- b ]咔唑的光物理性质可以通过改变核心中外围取代基的电子性质来简单地调节。
更新日期:2020-03-19
中文翻译:

通过TFA促进的硫代内酯,2-羟基-β-硝基苯乙烯和吲哚的级联反应有效获得荧光苯并呋喃[3,2-b]咔唑
建立了TFA促进的高效硫磺化物,2-羟基-β-硝基苯乙烯和吲哚的级联环化反应。该反应导致构建了一组不同的荧光苯并呋喃[3,2- b ]咔唑类化合物,并在一锅转化中生成了七个新键和两个新环。提出了该反应的合理机制。此外,观察到苯并呋喃并[3,2- b ]咔唑的光物理性质可以通过改变核心中外围取代基的电子性质来简单地调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号