European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.ejmech.2020.112173 Macarena Martínez-Bailén , Ana T. Carmona , Francesca Cardona , Camilla Matassini , Andrea Goti , Moemi Kubo , Atsushi Kato , Inmaculada Robina , Antonio J. Moreno-Vargas
|
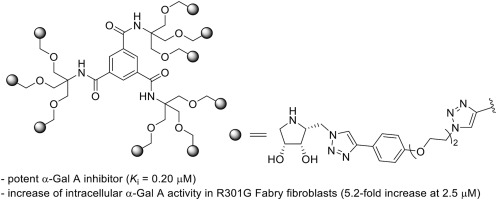
The synthesis of a chemical library of multimeric pyrrolidine-based iminosugars by incorporation of three pairs of epimeric pyrrolidine-azides into different alkyne scaffolds via CuAAC is presented. The new multimers were evaluated as inhibitors of two important therapeutic enzymes, human α-galactosidase A (α-Gal A) and lysosomal β-glucocerebrosidase (GCase). Structure-activity relationships were established focusing on the iminosugar inhitope, the valency of the dendron and the linker between the inhitope and the central scaffold. Remarkable is the result obtained in the inhibition of α-Gal A, where one of the nonavalent compounds showed potent inhibition (0.20 μM, competitive inhibition), being a 375-fold more potent inhibitor than the monovalent reference. The potential of the best α-Gal A inhibitors to act as pharmacological chaperones was analyzed by evaluating their ability to increase the activity of this enzyme in R301G fibroblasts from patients with Fabry disease, a genetic disorder related with a reduced activity of α-Gal A. The best enzyme activity enhancement was obtained for the same nonavalent compound, which increased 5.2-fold the activity of the misfolded enzyme at 2.5 μM, what constitutes the first example of a multivalent α-Gal A activity enhancer of potential interest in the treatment of Fabry disease.
中文翻译:

人β-葡萄糖脑苷脂酶和α-半乳糖苷酶的多聚吡咯烷亚氨基糖抑制剂的合成:法布里病多价酶活性增强剂的第一个实例
通过将三对对映体吡咯烷-叠氮化物通过以下方式掺入不同的炔烃骨架中,合成基于多吡咯烷的亚氨基糖的化学文库介绍了CuAAC。新的多聚体被评估为两种重要治疗酶的抑制剂,即人α-半乳糖苷酶A(α-GalA)和溶酶体β-葡萄糖脑苷脂酶(GCase)。建立了结构-活性关系,重点是亚氨基糖的抗原决定簇,树突的化合价以及抗原决定簇和中央支架之间的连接基。显着的是在抑制α-GalA时获得的结果,其中一种非价化合物显示出有效的抑制作用(0.20μM,竞争性抑制作用),是比单价参照物强375倍的有效抑制剂。通过评估其在Fabry病患者的R301G成纤维细胞中增加该酶活性的能力,分析了最佳α-GalA抑制剂作为药理伴侣的潜力,



























 京公网安备 11010802027423号
京公网安备 11010802027423号