当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silver-Catalyzed Activation of Terminal Alkynes for Synthesizing Nitrogen-Containing Molecules.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-02-20 , DOI: 10.1021/acs.accounts.9b00623 Paramasivam Sivaguru 1 , Shanshan Cao 1 , Kaki Raveendra Babu 1 , Xihe Bi 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-02-20 , DOI: 10.1021/acs.accounts.9b00623 Paramasivam Sivaguru 1 , Shanshan Cao 1 , Kaki Raveendra Babu 1 , Xihe Bi 1
Affiliation
|
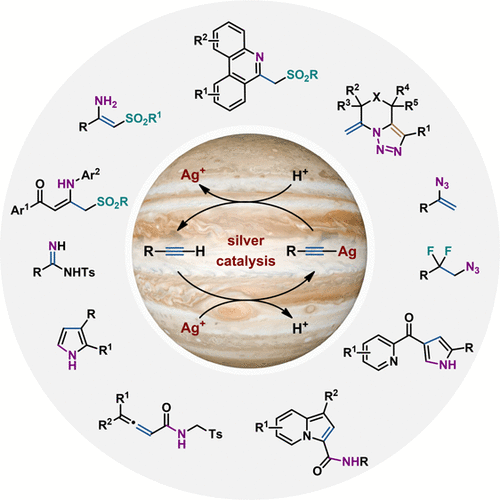
Alkynes are one of the most abundant chemicals in organic chemistry, and therefore the development of catalytic reactions to transform alkynes into other useful functionalities is of great value. In recent decades, extraordinary advances have been made in this area with transition-metal catalysis, and silver-based reagents are ideal for the activation of alkynes. This high reactivity is probably due to the superior π-Lewis acidic, carbophilic behavior of silver(I), allowing it to selectively activate carbon-carbon triple bonds (C≡C) through the formation of a silver-π complex. Within this field, we have been interested in the activation and subsequent reactions of readily accessible terminal alkynes for the synthesis of nitrogen-containing compounds, which has generally received less attention than methods involving internal alkynes. This is possibly due to the lack of suitable reactive reaction partners that are compatible under transition metals. Therefore, a thorough understanding of the factors that influence homogeneous silver catalysis and the identification of the appropriate reaction partners can provide a powerful platform for designing more efficient silver-catalyzed reactions of terminal alkynes. In this context, we envisioned that using readily available, environmentally benign, and inexpensive trimethylsilyl azide (TMSN3) or an isocyanide as the nitrogen-donor would be the key to develop novel reactions of terminal alkynes.This Account describes our efforts since 2013 toward the development of novel silver-catalyzed tandem reactions of terminal alkynes with either TMSN3 or isocyanides for the assembly of various nitrogen-containing compounds. The first section of this Account discusses the initial developments in the silver-catalyzed hydroazidation of terminal alkynes with TMSN3 and the subsequent advances made in our laboratory. We first describe the discovery and experimental and computational mechanistic investigations of silver-catalyzed hydroazidation reactions, which is the most efficient strategy reported to date for accessing vinyl azides. Mechanistic study of this hydroazidation reaction provides an alternative activation mode for terminal alkyne conversion in transition metal catalysis. We then present the chemistry of in situ generated vinyl azides, including one-pot tandem radical addition/cyclization or migration reactions of terminal alkynes to access a variety of nitrogen-containing molecules. Finally, we discuss the one-pot, multistep tandem hydroazidation and 1,2-azide migratory gem-difluorination of terminal alkynes for the synthesis of β-difluorinated alkyl azides. The second section describes the silver-catalyzed coupling reactions between terminal alkynes and isocyanides, which offer a straightforward method for accessing synthetically useful building blocks, such as pyrroles, allenamides, benzofuran, vinyl sulfones, indazolines, propiolonitriles, and pyrazoles. The high efficiency, mild conditions, low cost, broad substrate scope, high chemo- and regioselectivity, step economy, and ecofriendliness of the developed approaches make them attractive and practical. The progress in this area provides guiding principles for designing new reactions of terminal alkynes that can be extended to various nitrogen-containing molecules of interest to medicinal and materials chemists.
中文翻译:

末端炔烃的银催化活化,用于合成含氮分子。
炔烃是有机化学中最丰富的化学物质之一,因此开发将炔烃转化为其他有用功能的催化反应非常有价值。近几十年来,过渡金属催化在这一领域取得了非凡的进步,而基于银的试剂是炔烃活化的理想选择。这种高反应性可能是由于银(I)具有出色的π-路易斯酸性和嗜碳性,从而使其能够通过形成银-π络合物选择性激活碳-碳三键(C≡C)。在该领域中,我们对易于获得的末端炔烃的活化和随后的反应感兴趣,以用于合成含氮化合物,该方法通常不如涉及内部炔烃的方法受到关注。这可能是由于缺乏在过渡金属下相容的合适的反应性反应伙伴。因此,对影响均相银催化的因素的透彻了解和适当反应伙伴的识别可以为设计更高效的末端炔烃银催化反应提供强大的平台。在此背景下,我们设想使用易于获得,对环境无害且廉价的三甲基甲硅烷基叠氮化物(TMSN3)或异氰酸酯作为氮供体将是开发末端炔烃新反应的关键。此帐户描述了我们自2013年以来为新型炔烃末端炔与TMSN3或异氰酸酯的新型银催化串联反应的发展,用于组装各种含氮化合物。该帐户的第一部分讨论了末端炔烃与TMSN3的银催化加氢叠氮反应的初步进展以及我们实验室的后续进展。我们首先描述银催化加氢叠氮化反应的发现以及实验和计算机理的研究,这是迄今为止报道的最有效的访问乙烯基叠氮化物的策略。该加氢叠氮化反应的机理研究为过渡金属催化中末端炔烃转化提供了另一种活化方式。然后,我们介绍了原位生成的乙烯基叠氮化物的化学性质,包括一锅串连式自由基加成/环化或末端炔烃的迁移反应,以访问各种含氮分子。最后,我们讨论一锅多步串联水合叠氮化和 末端炔烃的2-叠氮化物迁移性宝石-二氟化反应,用于合成β-二氟化烷基叠氮化物。第二部分描述了末端炔烃与异氰酸酯之间的银催化偶联反应,这为获得合成有用的结构单元(例如吡咯,烯丙酰胺,苯并呋喃,乙烯基砜,吲唑啉,丙腈和吡唑)提供了直接的方法。所开发方法的高效,温和的条件,低成本,广泛的底物范围,高的化学和区域选择性,步骤经济性和环保性使它们具有吸引力和实用性。该领域的进展为设计末端炔烃的新反应提供了指导原则,这些末端炔烃可以扩展到医学和材料化学家感兴趣的各种含氮分子。
更新日期:2020-02-21
中文翻译:

末端炔烃的银催化活化,用于合成含氮分子。
炔烃是有机化学中最丰富的化学物质之一,因此开发将炔烃转化为其他有用功能的催化反应非常有价值。近几十年来,过渡金属催化在这一领域取得了非凡的进步,而基于银的试剂是炔烃活化的理想选择。这种高反应性可能是由于银(I)具有出色的π-路易斯酸性和嗜碳性,从而使其能够通过形成银-π络合物选择性激活碳-碳三键(C≡C)。在该领域中,我们对易于获得的末端炔烃的活化和随后的反应感兴趣,以用于合成含氮化合物,该方法通常不如涉及内部炔烃的方法受到关注。这可能是由于缺乏在过渡金属下相容的合适的反应性反应伙伴。因此,对影响均相银催化的因素的透彻了解和适当反应伙伴的识别可以为设计更高效的末端炔烃银催化反应提供强大的平台。在此背景下,我们设想使用易于获得,对环境无害且廉价的三甲基甲硅烷基叠氮化物(TMSN3)或异氰酸酯作为氮供体将是开发末端炔烃新反应的关键。此帐户描述了我们自2013年以来为新型炔烃末端炔与TMSN3或异氰酸酯的新型银催化串联反应的发展,用于组装各种含氮化合物。该帐户的第一部分讨论了末端炔烃与TMSN3的银催化加氢叠氮反应的初步进展以及我们实验室的后续进展。我们首先描述银催化加氢叠氮化反应的发现以及实验和计算机理的研究,这是迄今为止报道的最有效的访问乙烯基叠氮化物的策略。该加氢叠氮化反应的机理研究为过渡金属催化中末端炔烃转化提供了另一种活化方式。然后,我们介绍了原位生成的乙烯基叠氮化物的化学性质,包括一锅串连式自由基加成/环化或末端炔烃的迁移反应,以访问各种含氮分子。最后,我们讨论一锅多步串联水合叠氮化和 末端炔烃的2-叠氮化物迁移性宝石-二氟化反应,用于合成β-二氟化烷基叠氮化物。第二部分描述了末端炔烃与异氰酸酯之间的银催化偶联反应,这为获得合成有用的结构单元(例如吡咯,烯丙酰胺,苯并呋喃,乙烯基砜,吲唑啉,丙腈和吡唑)提供了直接的方法。所开发方法的高效,温和的条件,低成本,广泛的底物范围,高的化学和区域选择性,步骤经济性和环保性使它们具有吸引力和实用性。该领域的进展为设计末端炔烃的新反应提供了指导原则,这些末端炔烃可以扩展到医学和材料化学家感兴趣的各种含氮分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号