当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-Facet Dominant Anatase TiO2 (101) and (001) Model Catalysts to Elucidate the Active Sites for Alkanol Dehydration
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acscatal.9b04654 Fan Lin 1 , Yuan Chen 1 , Lu Zhang 1 , Donghai Mei 1 , Libor Kovarik 1 , Berlin Sudduth 2 , Huamin Wang 1 , Feng Gao 1 , Yong Wang 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acscatal.9b04654 Fan Lin 1 , Yuan Chen 1 , Lu Zhang 1 , Donghai Mei 1 , Libor Kovarik 1 , Berlin Sudduth 2 , Huamin Wang 1 , Feng Gao 1 , Yong Wang 1, 2
Affiliation
|
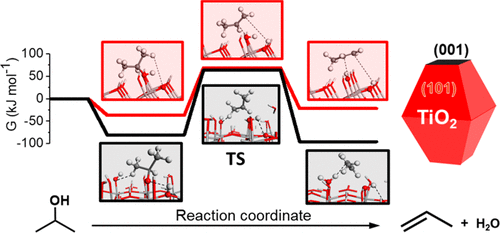
Alkanol dehydration on Lewis acid–base pairs of transition metal oxide catalysts is a reaction of importance in oxygen removal from biomass-derived feedstocks and their conversion to chemicals in general. However, catalysts with a high degree of structural heterogeneity, such as commercial TiO2 powders, are not well-suited to establish rigorous structure–function relationships at an atomic level. Here, we provide compelling evidence for the effects of surface orientation of TiO2 catalyst on elimination reactions of alcohols. Two anatase titania model catalysts, with preferential exposure of (101) and (001) facets, were synthesized and studied for 2-propanol dehydration using kinetic, isotopic, microscopic, and spectroscopic measurements, coupled with DFT calculations. Surface Lewis acid sites were found to be active for 2-propanol dehydration, and (101) facets are more reactive than (001) facets under the reaction conditions studied. On both anatase surfaces, 2-propanol was found to dehydrate via concerted E2 elimination pathways, but with different initial states and thus also different intrinsic activation barriers. Molecular 2-propanol dehydration dominates on TiO2 (101) while on TiO2 (001), 2-propanol simultaneously converts to more stable 2-propoxide before dehydration, which then requires higher activation energies for E2 elimination.
中文翻译:

单-Facet优势锐钛矿TiO 2(101)和(001)模型催化剂,以阐明烷醇脱水的活性位
在路易斯酸-碱过渡金属氧化物催化剂对上进行的醇脱水是一种重要的反应,它对从生物质衍生的原料中除氧以及通常将其转化为化学物质具有重要意义。但是,具有高度结构异质性的催化剂(例如商用TiO 2粉末)不适合在原子水平上建立严格的结构-功能关系。在这里,我们为TiO 2表面取向的影响提供了令人信服的证据消除醇反应的催化剂。合成了两种具有优先暴露(101)和(001)面的锐钛型二氧化钛模型催化剂,并使用动力学,同位素,显微镜和光谱测量以及DFT计算研究了2-丙醇脱水。发现表面路易斯酸位点对2-丙醇脱水具有活性,并且在研究的反应条件下,(101)晶面比(001)晶面更具活性。在两个锐钛矿表面上,发现2-丙醇通过协同的E2消除途径脱水,但具有不同的初始状态,因此也具有不同的固有活化屏障。分子2-丙醇脱水在TiO 2(101)上占优势,而在TiO 2上 (001),2-丙醇在脱水前同时转化为更稳定的2-丙氧基,这需要更高的活化能来消除E2。
更新日期:2020-03-21
中文翻译:

单-Facet优势锐钛矿TiO 2(101)和(001)模型催化剂,以阐明烷醇脱水的活性位
在路易斯酸-碱过渡金属氧化物催化剂对上进行的醇脱水是一种重要的反应,它对从生物质衍生的原料中除氧以及通常将其转化为化学物质具有重要意义。但是,具有高度结构异质性的催化剂(例如商用TiO 2粉末)不适合在原子水平上建立严格的结构-功能关系。在这里,我们为TiO 2表面取向的影响提供了令人信服的证据消除醇反应的催化剂。合成了两种具有优先暴露(101)和(001)面的锐钛型二氧化钛模型催化剂,并使用动力学,同位素,显微镜和光谱测量以及DFT计算研究了2-丙醇脱水。发现表面路易斯酸位点对2-丙醇脱水具有活性,并且在研究的反应条件下,(101)晶面比(001)晶面更具活性。在两个锐钛矿表面上,发现2-丙醇通过协同的E2消除途径脱水,但具有不同的初始状态,因此也具有不同的固有活化屏障。分子2-丙醇脱水在TiO 2(101)上占优势,而在TiO 2上 (001),2-丙醇在脱水前同时转化为更稳定的2-丙氧基,这需要更高的活化能来消除E2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号