Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2020-02-20 , DOI: 10.1016/s0140-6736(20)30258-0 Michael D Hill 1 , Mayank Goyal 1 , Bijoy K Menon 1 , Raul G Nogueira 2 , Ryan A McTaggart 3 , Andrew M Demchuk 1 , Alexandre Y Poppe 4 , Brian H Buck 5 , Thalia S Field 6 , Dar Dowlatshahi 7 , Brian A van Adel 8 , Richard H Swartz 9 , Ruchir A Shah 10 , Eric Sauvageau 11 , Charlotte Zerna 1 , Johanna M Ospel 1 , Manish Joshi 1 , Mohammed A Almekhlafi 1 , Karla J Ryckborst 1 , Mark W Lowerison 12 , Kathy Heard 13 , David Garman 13 , Diogo Haussen 2 , Shawna M Cutting 3 , Shelagh B Coutts 1 , Daniel Roy 4 , Jeremy L Rempel 5 , Axel Cr Rohr 6 , Daniela Iancu 14 , Demetrios J Sahlas 8 , Amy Y X Yu 9 , Thomas G Devlin 10 , Ricardo A Hanel 11 , Volker Puetz 15 , Frank L Silver 16 , Bruce C V Campbell 17 , René Chapot 18 , Jeanne Teitelbaum 19 , Jennifer L Mandzia 20 , Timothy J Kleinig 21 , David Turkel-Parrella 22 , Donald Heck 23 , Michael E Kelly 24 , Aditya Bharatha 25 , Oh Young Bang 26 , Ashutosh Jadhav 27 , Rishi Gupta 28 , Donald F Frei 29 , Jason W Tarpley 30 , Cameron G McDougall 31 , Staffan Holmin 32 , Joung-Ho Rha 33 , Ajit S Puri 34 , Marie-Christine Camden 35 , Götz Thomalla 36 , Hana Choe 37 , Stephen J Phillips 38 , Joseph L Schindler 39 , John Thornton 40 , Simon Nagel 41 , Ji Hoe Heo 42 , Sung-Il Sohn 43 , Marios-Nikos Psychogios 44 , Ronald F Budzik 45 , Sidney Starkman 46 , Coleman O Martin 47 , Paul A Burns 48 , Seán Murphy 49 , George A Lopez 50 , Joey English 51 , Michael Tymianski 13 ,
The Lancet ( IF 98.4 ) Pub Date : 2020-02-20 , DOI: 10.1016/s0140-6736(20)30258-0 Michael D Hill 1 , Mayank Goyal 1 , Bijoy K Menon 1 , Raul G Nogueira 2 , Ryan A McTaggart 3 , Andrew M Demchuk 1 , Alexandre Y Poppe 4 , Brian H Buck 5 , Thalia S Field 6 , Dar Dowlatshahi 7 , Brian A van Adel 8 , Richard H Swartz 9 , Ruchir A Shah 10 , Eric Sauvageau 11 , Charlotte Zerna 1 , Johanna M Ospel 1 , Manish Joshi 1 , Mohammed A Almekhlafi 1 , Karla J Ryckborst 1 , Mark W Lowerison 12 , Kathy Heard 13 , David Garman 13 , Diogo Haussen 2 , Shawna M Cutting 3 , Shelagh B Coutts 1 , Daniel Roy 4 , Jeremy L Rempel 5 , Axel Cr Rohr 6 , Daniela Iancu 14 , Demetrios J Sahlas 8 , Amy Y X Yu 9 , Thomas G Devlin 10 , Ricardo A Hanel 11 , Volker Puetz 15 , Frank L Silver 16 , Bruce C V Campbell 17 , René Chapot 18 , Jeanne Teitelbaum 19 , Jennifer L Mandzia 20 , Timothy J Kleinig 21 , David Turkel-Parrella 22 , Donald Heck 23 , Michael E Kelly 24 , Aditya Bharatha 25 , Oh Young Bang 26 , Ashutosh Jadhav 27 , Rishi Gupta 28 , Donald F Frei 29 , Jason W Tarpley 30 , Cameron G McDougall 31 , Staffan Holmin 32 , Joung-Ho Rha 33 , Ajit S Puri 34 , Marie-Christine Camden 35 , Götz Thomalla 36 , Hana Choe 37 , Stephen J Phillips 38 , Joseph L Schindler 39 , John Thornton 40 , Simon Nagel 41 , Ji Hoe Heo 42 , Sung-Il Sohn 43 , Marios-Nikos Psychogios 44 , Ronald F Budzik 45 , Sidney Starkman 46 , Coleman O Martin 47 , Paul A Burns 48 , Seán Murphy 49 , George A Lopez 50 , Joey English 51 , Michael Tymianski 13 ,
Affiliation
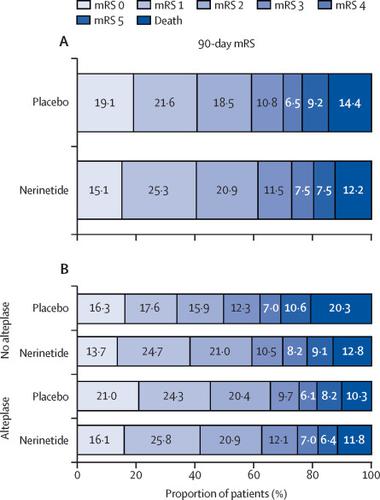
|
BACKGROUND
Nerinetide, an eicosapeptide that interferes with post-synaptic density protein 95, is a neuroprotectant that is effective in preclinical stroke models of ischaemia-reperfusion. In this trial, we assessed the efficacy and safety of nerinetide in human ischaemia-reperfusion that occurs with rapid endovascular thrombectomy in patients who had an acute ischaemic stroke.
METHODS
For this multicentre, double-blind, randomised, placebo-controlled study done in 48 acute care hospitals in eight countries, we enrolled patients with acute ischaemic stroke due to large vessel occlusion within a 12 h treatment window. Eligible patients were aged 18 years or older with a disabling ischaemic stroke at the time of randomisation, had been functioning independently in the community before the stroke, had an Alberta Stroke Program Early CT Score (ASPECTS) greater than 4, and vascular imaging showing moderate-to-good collateral filling, as determined by multiphase CT angiography. Patients were randomly assigned (1:1) to receive intravenous nerinetide in a single dose of 2·6 mg/kg, up to a maximum dose of 270 mg, on the basis of estimated or actual weight (if known) or saline placebo by use of a real-time, dynamic, internet-based, stratified randomised minimisation procedure. Patients were stratified by intravenous alteplase treatment and declared endovascular device choice. All trial personnel and patients were masked to sequence and treatment allocation. All patients underwent endovascular thrombectomy and received alteplase in usual care when indicated. The primary outcome was a favourable functional outcome 90 days after randomisation, defined as a modified Rankin Scale (mRS) score of 0-2. Secondary outcomes were measures of neurological disability, functional independence in activities of daily living, excellent functional outcome (mRS 0-1), and mortality. The analysis was done in the intention-to-treat population and adjusted for age, sex, baseline National Institutes of Health Stroke Scale score, ASPECTS, occlusion location, site, alteplase use, and declared first device. The safety population included all patients who received any amount of study drug. This trial is registered with ClinicalTrials.gov, NCT02930018.
FINDINGS
Between March 1, 2017, and Aug 12, 2019, 1105 patients were randomly assigned to receive nerinetide (n=549) or placebo (n=556). 337 (61·4%) of 549 patients with nerinetide and 329 (59·2%) of 556 with placebo achieved an mRS score of 0-2 at 90 days (adjusted risk ratio 1·04, 95% CI 0·96-1·14; p=0·35). Secondary outcomes were similar between groups. We observed evidence of treatment effect modification resulting in inhibition of treatment effect in patients receiving alteplase. Serious adverse events occurred equally between groups.
INTERPRETATION
Nerinetide did not improve the proportion of patients achieving good clinical outcomes after endovascular thrombectomy compared with patients receiving placebo.
FUNDING
Canadian Institutes for Health Research, Alberta Innovates, and NoNO.
中文翻译:

神经氨酸治疗急性缺血性卒中的疗效和安全性(ESCAPE-NA1):一项多中心,双盲,随机对照试验。
背景技术神经内酰胺是干扰突触后密度蛋白95的一种二十碳肽,是一种在脑缺血再灌注的临床前卒中模型中有效的神经保护剂。在该试验中,我们评估了神经氨酸肽在快速缺血性脑卒中患者快速血管内血栓切除术中发生的人类缺血-再灌注中的疗效和安全性。方法对于在8个国家/地区的48家急诊医院进行的这项多中心,双盲,随机,安慰剂对照研究,我们将因大血管阻塞导致的急性缺血性中风患者纳入了12小时的治疗窗口。符合条件的患者(年龄在18岁或以上)在随机分配时患有致残性缺血性中风,在中风前已在社区独立工作,阿尔伯塔省卒中计划早期CT评分(ASPECTS)大于4,并且血管成像显示中度到良好的侧支充盈,这是通过多相CT血管造影确定的。根据估计或实际体重(如果已知)或生理盐水安慰剂,将患者随机分配(1:1)以2·6 mg / kg的单次剂量接受最大剂量为270 mg的静脉注射神经氨酸治疗。使用基于互联网的实时,动态,分层随机最小化程序。通过静脉内阿替普酶治疗对患者进行分层,并宣布选择血管内器械。所有试验人员和患者都被掩盖了顺序和治疗分配。所有患者均行血管内血栓切除术,并在指示时接受阿替普酶常规治疗。主要结果是随机分组后90天获得了良好的功能性结果,定义为改良的Rankin量表(mRS)评分0-2。次要结果是神经功能障碍,日常生活活动中的功能独立性,出色的功能结果(mRS 0-1)和死亡率的量度。该分析在意向性治疗人群中进行,并针对年龄,性别,美国国立卫生研究院卒中量表评分基线,ASPECTS,阻塞位置,部位,阿替普酶的使用和宣布的第一种设备进行了调整。安全人群包括所有接受任何量研究药物的患者。该试验已在ClinicalTrials.gov(NCT02930018)上注册。结果在2017年3月1日至2019年8月12日之间,随机分配了1105例患者接受神经氨酸肽(n = 549)或安慰剂(n = 556)。549名神经氨酸肽患者中的337名(61·4%)和556名安慰剂患者中的329名(59·2%)在90天时的mRS评分为0-2(校正风险比1·04,95%CI 0·96- 1·14; p = 0·35)。各组之间的次要结局相似。我们观察到治疗效果改变的证据导致接受阿替普酶的患者的治疗效果受到抑制。严重不良事件在两组之间平均发生。解释与接受安慰剂的患者相比,神经内酰胺不能改善血管内血栓切除术后获得良好临床结果的患者比例。资助加拿大卫生研究院,艾伯塔省创新和NoNO。我们观察到治疗效果改变的证据导致接受阿替普酶的患者的治疗效果受到抑制。严重不良事件在两组之间平均发生。解释与接受安慰剂的患者相比,神经内酰胺不能改善血管内血栓切除术后获得良好临床结果的患者比例。资金加拿大卫生研究院,艾伯塔省创新和NoNO。我们观察到治疗效果改变的证据导致接受阿替普酶的患者的治疗效果受到抑制。两组之间严重不良事件的发生率均相等。解释与接受安慰剂的患者相比,神经内酰胺不能改善血管内血栓切除术后获得良好临床疗效的患者比例。资金加拿大卫生研究院,艾伯塔省创新和NoNO。
更新日期:2020-03-16
中文翻译:

神经氨酸治疗急性缺血性卒中的疗效和安全性(ESCAPE-NA1):一项多中心,双盲,随机对照试验。
背景技术神经内酰胺是干扰突触后密度蛋白95的一种二十碳肽,是一种在脑缺血再灌注的临床前卒中模型中有效的神经保护剂。在该试验中,我们评估了神经氨酸肽在快速缺血性脑卒中患者快速血管内血栓切除术中发生的人类缺血-再灌注中的疗效和安全性。方法对于在8个国家/地区的48家急诊医院进行的这项多中心,双盲,随机,安慰剂对照研究,我们将因大血管阻塞导致的急性缺血性中风患者纳入了12小时的治疗窗口。符合条件的患者(年龄在18岁或以上)在随机分配时患有致残性缺血性中风,在中风前已在社区独立工作,阿尔伯塔省卒中计划早期CT评分(ASPECTS)大于4,并且血管成像显示中度到良好的侧支充盈,这是通过多相CT血管造影确定的。根据估计或实际体重(如果已知)或生理盐水安慰剂,将患者随机分配(1:1)以2·6 mg / kg的单次剂量接受最大剂量为270 mg的静脉注射神经氨酸治疗。使用基于互联网的实时,动态,分层随机最小化程序。通过静脉内阿替普酶治疗对患者进行分层,并宣布选择血管内器械。所有试验人员和患者都被掩盖了顺序和治疗分配。所有患者均行血管内血栓切除术,并在指示时接受阿替普酶常规治疗。主要结果是随机分组后90天获得了良好的功能性结果,定义为改良的Rankin量表(mRS)评分0-2。次要结果是神经功能障碍,日常生活活动中的功能独立性,出色的功能结果(mRS 0-1)和死亡率的量度。该分析在意向性治疗人群中进行,并针对年龄,性别,美国国立卫生研究院卒中量表评分基线,ASPECTS,阻塞位置,部位,阿替普酶的使用和宣布的第一种设备进行了调整。安全人群包括所有接受任何量研究药物的患者。该试验已在ClinicalTrials.gov(NCT02930018)上注册。结果在2017年3月1日至2019年8月12日之间,随机分配了1105例患者接受神经氨酸肽(n = 549)或安慰剂(n = 556)。549名神经氨酸肽患者中的337名(61·4%)和556名安慰剂患者中的329名(59·2%)在90天时的mRS评分为0-2(校正风险比1·04,95%CI 0·96- 1·14; p = 0·35)。各组之间的次要结局相似。我们观察到治疗效果改变的证据导致接受阿替普酶的患者的治疗效果受到抑制。严重不良事件在两组之间平均发生。解释与接受安慰剂的患者相比,神经内酰胺不能改善血管内血栓切除术后获得良好临床结果的患者比例。资助加拿大卫生研究院,艾伯塔省创新和NoNO。我们观察到治疗效果改变的证据导致接受阿替普酶的患者的治疗效果受到抑制。严重不良事件在两组之间平均发生。解释与接受安慰剂的患者相比,神经内酰胺不能改善血管内血栓切除术后获得良好临床结果的患者比例。资金加拿大卫生研究院,艾伯塔省创新和NoNO。我们观察到治疗效果改变的证据导致接受阿替普酶的患者的治疗效果受到抑制。两组之间严重不良事件的发生率均相等。解释与接受安慰剂的患者相比,神经内酰胺不能改善血管内血栓切除术后获得良好临床疗效的患者比例。资金加拿大卫生研究院,艾伯塔省创新和NoNO。











































 京公网安备 11010802027423号
京公网安备 11010802027423号