Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.jorganchem.2020.121195 Chang Cao , Guoliang Mao , Jun Cheng , Xuebing Leng , Liang Deng
|
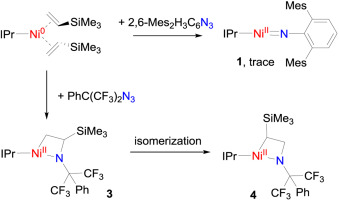
The reactions of three-coordinate iron(0) and cobalt(0) alkene complexes (NHC)M(η2-CH2CHSiMe3)2 (NHC = N-heterocyclic carbene, M = Fe, Co) with organic azides proved an effective way for the preparation of iron and cobalt terminal imido complexes. The relevant reactions of nickel(0) alkene complex (NHC)Ni(η2-CH2CHSiMe3)2 with organic azides, however, have remained unexplored. Herein, we report the study on the reactions of [(IPr)Ni(η2-CH2CHSiMe3)2] (IPr = 1,3-bis(2′,6′-diisopropylphenyl)imidazole-2-ylidene) with organic azides. The interaction of [(IPr)Ni(η2-CH2CHSiMe3)2] with 2,6-dimesitylphenyl azide (DmpN3) produced the two-coordinate imido complex [(IPr)Ni(NDmp)] (1) in a trace amount with the starting materials being largely remained. The reaction of [(IPr)Ni(η2-CH2CHSiMe3)2] with 2,6-diisopropylphenyl azide (DippN3) gave IPrNNNDipp (2) as the only isolable product. The interaction of [(IPr)Ni(η2-CH2CHSiMe3)2] with Ph(F3C)2CN3 produced an azanickelacyclobutane compound [(IPr)Ni(κ-C,N–N(C(CF3)2Ph)CH(SiMe3)CH2)] (3) in high yield. The different products formed in these reactions reflect the influence of the steric and electronic nature of organic azides on reaction outcomes. Further study on the azanickelacyclobutane complex 3 indicated that it can convert into a new azanickelacyclobutane [(IPr)Ni(κ-C,N–N(C(CF3)2Ph)CH2CHSiMe3)] (4). A nickel(II) amido alkyl complex [(IPr)Ni(NHC(CF3)2Ph)(CH2CHNC(CF3)2Ph)] (5) was also isolated from the decomposition reaction of 3.
中文翻译:

双(乙烯基三甲基硅烷)镍(0)N-杂环卡宾配合物与有机叠氮化物的反应
的三维坐标铁(0)和钴(0)配合物的烯烃(NHC)M(η反应2 -CH 2 CHSiMe 3)2(NHC = Ñ -杂环卡宾,M =铁,钴)与有机叠氮化物的证明制备铁钴末端亚胺配合物的有效途径。镍(0)烯烃配合物(NHC)的Ni(η的相关反应2 -CH 2 CHSiMe 3)2与有机叠氮化物,然而,仍然未开发。在本文中,我们报道了在[(IPR)Ni的反应的研究(η 2 -CH 2 CHSiMe 3)2](IPr = 1,3-双(2',6'-二异丙基苯基)咪唑-2-亚烷基)与有机叠氮化物。的相互作用[(IPR)的Ni(η 2 -CH 2 CHSiMe 3)2 ]与2,6- dimesitylphenyl叠氮化物(DmpN 3)中产生的两个坐标酰亚胺配合物[(IPR)的Ni(NDMP)](1)在微量残留,起始原料大量保留。的反应[(IPR)的Ni(η 2 -CH 2 CHSiMe 3)2 ]与2,6-二异丙基叠氮化物(DippN 3)得到IPrNNNDipp(2)作为唯一的可分离产物。[(IPR)Ni的相互作用(η 2 -CH 2 CHSiMe3)2 ]与Ph(F 3 C)2 CN 3生成了氮杂氮杂环丁烷化合物[(IPr)Ni(κ-C, N – N(C(CF 3)2 Ph)CH(SiMe 3)CH 2)]] 3)高产。在这些反应中形成的不同产物反映了有机叠氮化物的空间和电子性质对反应结果的影响。对氮杂镍环丁烷络合物3的进一步研究表明,它可以转化为新的氮杂镍环丁烷[(IPr)Ni(κ-C, N – N(C(CF 3)2 Ph)CH 2 CHSiMe 3)](4)。从3的分解反应中也分离出镍(II)酰胺基烷基络合物[(IPr)Ni(NHC(CF 3)2 Ph)(CH 2 CHNC(CF 3)2 Ph)](5)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号