当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Separation and Cytotoxicity of Tau are Modulated by Protein Disulfide Isomerase and S-nitrosylation of this Molecular Chaperone.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.jmb.2020.02.013 Kan Wang 1 , Jia-Qi Liu 1 , Tao Zhong 1 , Xiao-Ling Liu 1 , Yan Zeng 1 , Xinhua Qiao 2 , Ting Xie 2 , Yuzhe Chen 2 , Ying-Ying Gao 1 , Bo Tang 3 , Jia Li 4 , Jun Zhou 4 , Dai-Wen Pang 3 , Jie Chen 1 , Chang Chen 2 , Yi Liang 1
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.jmb.2020.02.013 Kan Wang 1 , Jia-Qi Liu 1 , Tao Zhong 1 , Xiao-Ling Liu 1 , Yan Zeng 1 , Xinhua Qiao 2 , Ting Xie 2 , Yuzhe Chen 2 , Ying-Ying Gao 1 , Bo Tang 3 , Jia Li 4 , Jun Zhou 4 , Dai-Wen Pang 3 , Jie Chen 1 , Chang Chen 2 , Yi Liang 1
Affiliation
|
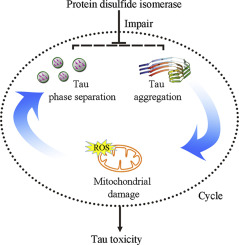
Cells have evolved molecular chaperones that modulate phase separation and misfolding of amyloidogenic proteins to prevent neurodegenerative diseases. Protein disulfide isomerase (PDI), mainly located at the endoplasmic reticulum and also present in the cytosol, acts as both an enzyme and a molecular chaperone. PDI is observed to be S-nitrosylated in the brain of Alzheimer's disease patients, but the mechanism has remained elusive. We herein report that both wild-type PDI and its quadruple cysteine mutant only having chaperone activity, significantly inhibit pathological phosphorylation and abnormal aggregation of Tau in cells, and significantly decrease the mitochondrial damage and Tau cytotoxicity resulting from Tau aberrant aggregation, highlighting the chaperone property of PDI. More importantly, we show that wild-type PDI is selectively recruited by liquid droplets of Tau, which significantly inhibits phase separation and stress granule formation of Tau, whereas S-nitrosylation of PDI abrogates the recruitment and inhibition. These findings demonstrate how phase separation of Tau is physiologically regulated by PDI and how S-nitrosylation of PDI, a perturbation in this regulation, leads to disease.
中文翻译:

Tau的相分离和细胞毒性受蛋白质二硫键异构酶和该分子伴侣的S-亚硝基化影响。
细胞已经进化出了分子伴侣,它们可以调节淀粉样蛋白的相分离和错误折叠,从而预防神经退行性疾病。蛋白质二硫键异构酶(PDI)主要位于内质网,也存在于细胞质中,既是酶又是分子伴侣。在阿尔茨海默氏病患者的大脑中发现PDI是S-亚硝基化的,但该机制仍然难以捉摸。我们在此报告,野生型PDI及其仅具有伴侣活性的四倍半胱氨酸突变体均显着抑制细胞内Tau的病理磷酸化和异常聚集,并显着降低Tau异常聚集导致的线粒体损伤和Tau细胞毒性,从而突出了伴侣的特性。 PDI。更重要的是,我们显示野生型PDI被Tau的液滴选择性地募集,这显着抑制Tau的相分离和应力颗粒的形成,而PDI的S-亚硝基化则消除了募集和抑制。这些发现表明,Tau的相分离是如何在生理上受PDI调节的,以及PDI的S-亚硝基化(在该调节中的扰动)如何导致疾病。
更新日期:2020-02-20
中文翻译:

Tau的相分离和细胞毒性受蛋白质二硫键异构酶和该分子伴侣的S-亚硝基化影响。
细胞已经进化出了分子伴侣,它们可以调节淀粉样蛋白的相分离和错误折叠,从而预防神经退行性疾病。蛋白质二硫键异构酶(PDI)主要位于内质网,也存在于细胞质中,既是酶又是分子伴侣。在阿尔茨海默氏病患者的大脑中发现PDI是S-亚硝基化的,但该机制仍然难以捉摸。我们在此报告,野生型PDI及其仅具有伴侣活性的四倍半胱氨酸突变体均显着抑制细胞内Tau的病理磷酸化和异常聚集,并显着降低Tau异常聚集导致的线粒体损伤和Tau细胞毒性,从而突出了伴侣的特性。 PDI。更重要的是,我们显示野生型PDI被Tau的液滴选择性地募集,这显着抑制Tau的相分离和应力颗粒的形成,而PDI的S-亚硝基化则消除了募集和抑制。这些发现表明,Tau的相分离是如何在生理上受PDI调节的,以及PDI的S-亚硝基化(在该调节中的扰动)如何导致疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号