当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conditional Disorder in Small Heat-shock Proteins.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.jmb.2020.02.003 T Reid Alderson 1 , Jinfa Ying 2 , Ad Bax 2 , Justin L P Benesch 3 , Andrew J Baldwin 3
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.jmb.2020.02.003 T Reid Alderson 1 , Jinfa Ying 2 , Ad Bax 2 , Justin L P Benesch 3 , Andrew J Baldwin 3
Affiliation
|
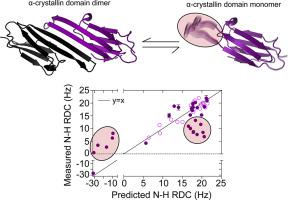
Small heat-shock proteins (sHSPs) are molecular chaperones that respond to cellular stresses to combat protein aggregation. HSP27 is a critical human sHSP that forms large, dynamic oligomers whose quaternary structures and chaperone activities depend on environmental factors. Upon exposure to cellular stresses, such as heat shock or acidosis, HSP27 oligomers can dissociate into dimers and monomers, which leads to significantly enhanced chaperone activity. The structured core of the protein, the α-crystallin domain (ACD), forms dimers and can prevent the aggregation of substrate proteins to a similar degree as the full-length protein. When the ACD dimer dissociates into monomers, it partially unfolds and exhibits enhanced activity. Here, we used solution-state NMR spectroscopy to characterize the structure and dynamics of the HSP27 ACD monomer. Web show that the monomer is stabilized at low pH and that its backbone chemical shifts, 15N relaxation rates, and 1H-15N residual dipolar couplings suggest structural changes and rapid motions in the region responsible for dimerization. By analyzing the solvent accessible and buried surface areas of sHSP structures in the context of a database of dimers that are known to dissociate into disordered monomers, we predict that ACD dimers from sHSPs across all kingdoms of life may partially unfold upon dissociation. We propose a general model in which conditional disorder-the partial unfolding of ACDs upon monomerization-is a common mechanism for sHSP activity.
中文翻译:

小热休克蛋白的条件性紊乱。
小热休克蛋白 (sHSP) 是响应细胞应激以对抗蛋白质聚集的分子伴侣。 HSP27 是一种关键的人类 sHSP,可形成大型动态寡聚物,其四级结构和伴侣活性取决于环境因素。当暴露于热休克或酸中毒等细胞应激时,HSP27 寡聚体可以解离成二聚体和单体,从而导致伴侣活性显着增强。该蛋白质的结构核心,α-晶状体蛋白结构域 (ACD),形成二聚体,可以防止底物蛋白质的聚集,其程度与全长蛋白质相似。当 ACD 二聚体解离成单体时,它部分展开并表现出增强的活性。在这里,我们使用溶液态核磁共振波谱来表征 HSP27 ACD 单体的结构和动力学。 Web 显示单体在低 pH 下稳定,其主链化学位移、15N 弛豫速率和 1H-15N 残余偶极耦合表明负责二聚化的区域发生结构变化和快速运动。通过在已知解离成无序单体的二聚体数据库中分析 sHSP 结构的溶剂可及和埋藏表面积,我们预测来自所有生命王国的 sHSP 的 ACD 二聚体可能在解离后部分展开。我们提出了一个通用模型,其中条件障碍(单体化时 ACD 的部分展开)是 sHSP 活性的常见机制。
更新日期:2020-02-17
中文翻译:

小热休克蛋白的条件性紊乱。
小热休克蛋白 (sHSP) 是响应细胞应激以对抗蛋白质聚集的分子伴侣。 HSP27 是一种关键的人类 sHSP,可形成大型动态寡聚物,其四级结构和伴侣活性取决于环境因素。当暴露于热休克或酸中毒等细胞应激时,HSP27 寡聚体可以解离成二聚体和单体,从而导致伴侣活性显着增强。该蛋白质的结构核心,α-晶状体蛋白结构域 (ACD),形成二聚体,可以防止底物蛋白质的聚集,其程度与全长蛋白质相似。当 ACD 二聚体解离成单体时,它部分展开并表现出增强的活性。在这里,我们使用溶液态核磁共振波谱来表征 HSP27 ACD 单体的结构和动力学。 Web 显示单体在低 pH 下稳定,其主链化学位移、15N 弛豫速率和 1H-15N 残余偶极耦合表明负责二聚化的区域发生结构变化和快速运动。通过在已知解离成无序单体的二聚体数据库中分析 sHSP 结构的溶剂可及和埋藏表面积,我们预测来自所有生命王国的 sHSP 的 ACD 二聚体可能在解离后部分展开。我们提出了一个通用模型,其中条件障碍(单体化时 ACD 的部分展开)是 sHSP 活性的常见机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号