当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Pierced Lasso Topology Leptin has a Bolt on Dynamic Domain Composed by the Disordered Loops I and III.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.jmb.2020.01.035 Jens Danielsson 1 , Jeffrey Kenneth Noel 2 , Jennifer Michelle Simien 3 , Brendan Michael Duggan 4 , Mikael Oliveberg 1 , José Nelson Onuchic 5 , Patricia Ann Jennings 6 , Ellinor Haglund 3
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.jmb.2020.01.035 Jens Danielsson 1 , Jeffrey Kenneth Noel 2 , Jennifer Michelle Simien 3 , Brendan Michael Duggan 4 , Mikael Oliveberg 1 , José Nelson Onuchic 5 , Patricia Ann Jennings 6 , Ellinor Haglund 3
Affiliation
|
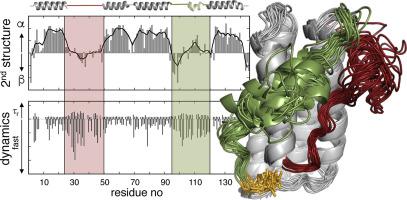
Leptin is an important signaling hormone, mostly known for its role in energy expenditure and satiety. Furthermore, leptin plays a major role in other proteinopathies, such as cancer, marked hyperphagia, impaired immune function, and inflammation. In spite of its biological relevance in human health, there are no NMR resonance assignments of the human protein available, obscuring high-resolution characterization of the soluble protein and/or its conformational dynamics, suggested as being important for receptor interaction and biological activity. Here, we report the nearly complete backbone resonance assignments of human leptin. Chemical shift-based secondary structure prediction confirms that in solution leptin forms a four-helix bundle including a pierced lasso topology. The conformational dynamics, determined on several timescales, show that leptin is monomeric, has a rigid four-helix scaffold, and a dynamic domain, including a transiently formed helix. The dynamic domain is anchored to the helical scaffold by a secondary hydrophobic core, pinning down the long loops of leptin to the protein body, inducing motional restriction without a well-defined secondary or tertiary hydrogen bond stabilized structure. This dynamic region is well suited for and may be involved in functional allosteric dynamics upon receptor binding.
中文翻译:

刺穿套索拓扑瘦素在由无序环 I 和 III 组成的动态域上有一个螺栓。
瘦素是一种重要的信号激素,因其在能量消耗和饱腹感中的作用而闻名。此外,瘦素在其他蛋白质病中发挥着重要作用,例如癌症、明显的食欲过盛、免疫功能受损和炎症。尽管其与人类健康具有生物学相关性,但没有可用的人类蛋白质的 NMR 共振归属,从而模糊了可溶性蛋白质和/或其构象动力学的高分辨率表征,而这被认为对受体相互作用和生物活性很重要。在这里,我们报告了人类瘦素几乎完整的主干共振分配。基于化学位移的二级结构预测证实,瘦素在溶液中形成四螺旋束,包括刺穿的套索拓扑。在多个时间尺度上确定的构象动力学表明,瘦素是单体,具有刚性的四螺旋支架和动态结构域,包括瞬时形成的螺旋。动态结构域通过二级疏水核心锚定在螺旋支架上,将瘦素的长环固定在蛋白体上,在没有明确的二级或三级氢键稳定结构的情况下诱导运动限制。该动态区域非常适合并可能参与受体结合后的功能变构动力学。
更新日期:2020-02-17
中文翻译:

刺穿套索拓扑瘦素在由无序环 I 和 III 组成的动态域上有一个螺栓。
瘦素是一种重要的信号激素,因其在能量消耗和饱腹感中的作用而闻名。此外,瘦素在其他蛋白质病中发挥着重要作用,例如癌症、明显的食欲过盛、免疫功能受损和炎症。尽管其与人类健康具有生物学相关性,但没有可用的人类蛋白质的 NMR 共振归属,从而模糊了可溶性蛋白质和/或其构象动力学的高分辨率表征,而这被认为对受体相互作用和生物活性很重要。在这里,我们报告了人类瘦素几乎完整的主干共振分配。基于化学位移的二级结构预测证实,瘦素在溶液中形成四螺旋束,包括刺穿的套索拓扑。在多个时间尺度上确定的构象动力学表明,瘦素是单体,具有刚性的四螺旋支架和动态结构域,包括瞬时形成的螺旋。动态结构域通过二级疏水核心锚定在螺旋支架上,将瘦素的长环固定在蛋白体上,在没有明确的二级或三级氢键稳定结构的情况下诱导运动限制。该动态区域非常适合并可能参与受体结合后的功能变构动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号