当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heme Oxygenase-1 in liver transplant ischemia-reperfusion injury: From bench-to-bedside.
Free Radical Biology and Medicine ( IF 7.4 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.freeradbiomed.2020.02.012 Hirofumi Hirao 1 , Kenneth J Dery 1 , Shoichi Kageyama 1 , Kojiro Nakamura 2 , Jerzy W Kupiec-Weglinski 1
Free Radical Biology and Medicine ( IF 7.4 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.freeradbiomed.2020.02.012 Hirofumi Hirao 1 , Kenneth J Dery 1 , Shoichi Kageyama 1 , Kojiro Nakamura 2 , Jerzy W Kupiec-Weglinski 1
Affiliation
|
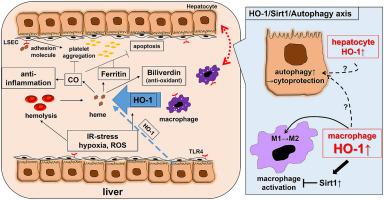
Hepatic ischemia-reperfusion injury (IRI), a major risk factor for early allograft dysfunction (EAD) and acute or chronic graft rejection, contributes to donor organ shortage for life-saving orthotopic liver transplantation (OLT). The graft injury caused by local ischemia (warm and/or cold) leads to parenchymal cell death and release of danger-associated molecular patterns (DAMPs), followed by reperfusion-triggered production of reactive oxygen species (ROS), activation of inflammatory cells, hepatocellular damage and ultimate organ failure. Heme oxygenase 1 (HO-1), a heat shock protein-32 induced under IR-stress, is an essential component of the cytoprotective mechanism in stressed livers. HO-1 regulates anti-inflammatory responses and may be crucial in the pathogenesis of chronic diseases, such as arteriosclerosis, hypertension, diabetes and steatosis. An emerging area of study is macrophage-derived HO-1 and its pivotal intrahepatic homeostatic function played in IRI-OLT. Indeed, ectopic hepatic HO-1 overexpression activates intracellular SIRT1/autophagy axis to serve as a key cellular self-defense mechanism in both mouse and human OLT recipients. Recent translational studies in rodents and human liver transplant patients provide novel insights into HO-1 mediated cytoprotection against sterile hepatic inflammation. In this review, we summarize the current bench-to-bedside knowledge on HO-1 molecular signaling and discuss their future therapeutic potential to mitigate IRI in OLT.
中文翻译:

肝移植缺血再灌注损伤中的血红素加氧酶 1:从工作台到床边。
肝缺血再灌注损伤 (IRI) 是早期同种异体移植物功能障碍 (EAD) 和急性或慢性移植物排斥反应的主要危险因素,导致挽救生命的原位肝移植 (OLT) 供体器官短缺。由局部缺血(热和/或冷)引起的移植物损伤导致实质细胞死亡和危险相关分子模式(DAMP)的释放,随后再灌注引发活性氧(ROS)的产生,炎症细胞的激活,肝细胞损伤和最终的器官衰竭。血红素加氧酶 1 (HO-1) 是一种在 IR 应激下诱导的热休克蛋白 32,是应激肝脏中细胞保护机制的重要组成部分。HO-1 调节抗炎反应,可能在慢性疾病的发病机制中起关键作用,如动脉硬化、高血压、糖尿病和脂肪变性。一个新兴的研究领域是巨噬细胞衍生的 HO-1 及其在 IRI-OLT 中发挥的关键肝内稳态功能。事实上,异位肝 HO-1 过表达激活细胞内 SIRT1/自噬轴,作为小鼠和人类 OLT 受体的关键细胞自我防御机制。最近在啮齿动物和人类肝移植患者中的转化研究为 HO-1 介导的细胞保护对抗无菌性肝脏炎症提供了新的见解。在这篇综述中,我们总结了当前关于 HO-1 分子信号传导的从实验室到床边的知识,并讨论了它们在 OLT 中减轻 IRI 的未来治疗潜力。异位肝 HO-1 过表达激活细胞内 SIRT1/自噬轴,作为小鼠和人类 OLT 受体的关键细胞自我防御机制。最近在啮齿动物和人类肝移植患者中的转化研究为 HO-1 介导的细胞保护对抗无菌性肝脏炎症提供了新的见解。在这篇综述中,我们总结了当前关于 HO-1 分子信号传导的从实验室到床边的知识,并讨论了它们在 OLT 中减轻 IRI 的未来治疗潜力。异位肝 HO-1 过表达激活细胞内 SIRT1/自噬轴,作为小鼠和人类 OLT 受体的关键细胞自我防御机制。最近在啮齿动物和人类肝移植患者中的转化研究为 HO-1 介导的细胞保护对抗无菌性肝脏炎症提供了新的见解。在这篇综述中,我们总结了当前关于 HO-1 分子信号传导的从实验室到床边的知识,并讨论了它们在 OLT 中减轻 IRI 的未来治疗潜力。
更新日期:2020-02-19
中文翻译:

肝移植缺血再灌注损伤中的血红素加氧酶 1:从工作台到床边。
肝缺血再灌注损伤 (IRI) 是早期同种异体移植物功能障碍 (EAD) 和急性或慢性移植物排斥反应的主要危险因素,导致挽救生命的原位肝移植 (OLT) 供体器官短缺。由局部缺血(热和/或冷)引起的移植物损伤导致实质细胞死亡和危险相关分子模式(DAMP)的释放,随后再灌注引发活性氧(ROS)的产生,炎症细胞的激活,肝细胞损伤和最终的器官衰竭。血红素加氧酶 1 (HO-1) 是一种在 IR 应激下诱导的热休克蛋白 32,是应激肝脏中细胞保护机制的重要组成部分。HO-1 调节抗炎反应,可能在慢性疾病的发病机制中起关键作用,如动脉硬化、高血压、糖尿病和脂肪变性。一个新兴的研究领域是巨噬细胞衍生的 HO-1 及其在 IRI-OLT 中发挥的关键肝内稳态功能。事实上,异位肝 HO-1 过表达激活细胞内 SIRT1/自噬轴,作为小鼠和人类 OLT 受体的关键细胞自我防御机制。最近在啮齿动物和人类肝移植患者中的转化研究为 HO-1 介导的细胞保护对抗无菌性肝脏炎症提供了新的见解。在这篇综述中,我们总结了当前关于 HO-1 分子信号传导的从实验室到床边的知识,并讨论了它们在 OLT 中减轻 IRI 的未来治疗潜力。异位肝 HO-1 过表达激活细胞内 SIRT1/自噬轴,作为小鼠和人类 OLT 受体的关键细胞自我防御机制。最近在啮齿动物和人类肝移植患者中的转化研究为 HO-1 介导的细胞保护对抗无菌性肝脏炎症提供了新的见解。在这篇综述中,我们总结了当前关于 HO-1 分子信号传导的从实验室到床边的知识,并讨论了它们在 OLT 中减轻 IRI 的未来治疗潜力。异位肝 HO-1 过表达激活细胞内 SIRT1/自噬轴,作为小鼠和人类 OLT 受体的关键细胞自我防御机制。最近在啮齿动物和人类肝移植患者中的转化研究为 HO-1 介导的细胞保护对抗无菌性肝脏炎症提供了新的见解。在这篇综述中,我们总结了当前关于 HO-1 分子信号传导的从实验室到床边的知识,并讨论了它们在 OLT 中减轻 IRI 的未来治疗潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号