当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Geniposide protects against sepsis-induced myocardial dysfunction through AMPKα-dependent pathway.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.freeradbiomed.2020.02.011 Peng Song 1 , Di-Fei Shen 1 , Yan-Yan Meng 1 , Chun-Yan Kong 1 , Xin Zhang 1 , Yu-Pei Yuan 1 , Ling Yan 1 , Qi-Zhu Tang 1 , Zhen-Guo Ma 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.freeradbiomed.2020.02.011 Peng Song 1 , Di-Fei Shen 1 , Yan-Yan Meng 1 , Chun-Yan Kong 1 , Xin Zhang 1 , Yu-Pei Yuan 1 , Ling Yan 1 , Qi-Zhu Tang 1 , Zhen-Guo Ma 1
Affiliation
|
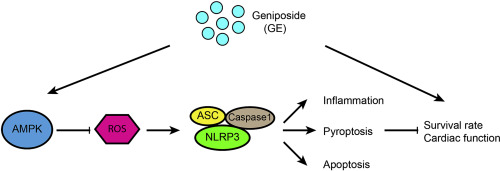
Uncontrolled inflammatory response and subsequent cardiomyocytes loss (apoptosis and pyroptosis) are closely involved in sepsis-induced myocardial dysfunction. Our previous study has found that geniposide (GE) can protect the murine hearts against obesity-induced inflammation. However, the effect of GE on sepsis-related cardiac dysfunction is still unknown. Mice were exposed to lipopolysaccharide (LPS) to generate sepsis-induced myocardial dysfunction. And 50 mg/kg GE was used to treat mice for consecutive 7 days. Our results showed that GE treatment significantly improved survival rate and cardiac function, and suppressed myocardial inflammatory response, as well as myocardial loss in LPS-treated mice. Those effects of GE were largely abolished in NOD-like receptor protein 3 (NLRP3)-deficient mice. Further detection revealed that the inhibition of NLRP3 inflammasome activation depended on the reduction of p47phox by GE. GE treatment restored the phosphorylation and activity of AMP-activated protein kinase α (AMPKα) in the hearts of sepsis mice, and knockout of AMPKα abolished the protection of GE against reactive oxygen species (ROS) accumulation, NLRP3 inflammasome activation and cardiomyocytes loss in sepsis mice. In conclusion, our findings revealed that GE activated AMPKα to suppress myocardial ROS accumulation, thus blocking NLRP3 inflammasome-mediated cardiomyocyte apoptosis and pyroptosis and improving cardiac function in mice with sepsis.
中文翻译:

ip子苷可通过AMPKα依赖性途径来预防败血症引起的心肌功能障碍。
脓毒症引起的心肌功能异常与失控的炎症反应以及随后的心肌细胞丢失(凋亡和焦磷酸化)密切相关。我们以前的研究发现子苷(GE)可以保护鼠类心脏免于肥胖引起的炎症。然而,GE对败血症相关的心脏功能障碍的影响仍然未知。将小鼠暴露于脂多糖(LPS),以产生败血症诱导的心肌功能障碍。50 mg / kg GE连续7天用于治疗小鼠。我们的结果表明,GE的治疗显着提高了存活率和心脏功能,并抑制了LPS治疗小鼠的心肌炎症反应以及心肌损失。在那些NOD样受体蛋白3(NLRP3)缺陷小鼠中,GE的这些作用在很大程度上被消除了。进一步的检测表明,NLRP3炎性体激活的抑制取决于GE对p47phox的减少。GE处理恢复败血症小鼠心脏中AMP活化蛋白激酶α(AMPKα)的磷酸化和活性,敲除AMPKα取消了GE对败血症中活性氧(ROS)积累,NLRP3炎症小体活化和心肌细胞丢失的保护作用老鼠。总之,我们的发现表明,GE激活AMPKα抑制心肌ROS的积累,从而阻断NLRP3炎性体介导的心肌细胞的凋亡和凋亡,并改善败血症小鼠的心脏功能。GE处理恢复败血症小鼠心脏中AMP活化蛋白激酶α(AMPKα)的磷酸化和活性,敲除AMPKα取消了GE对败血症中活性氧(ROS)积累,NLRP3炎症小体活化和心肌细胞丢失的保护作用老鼠。总之,我们的发现表明,GE激活AMPKα抑制心肌ROS的积累,从而阻断NLRP3炎性体介导的心肌细胞的凋亡和凋亡,并改善败血症小鼠的心脏功能。GE处理恢复败血症小鼠心脏中AMP活化蛋白激酶α(AMPKα)的磷酸化和活性,敲除AMPKα取消了GE对败血症中活性氧(ROS)积累,NLRP3炎症小体活化和心肌细胞丢失的保护作用老鼠。总之,我们的发现表明,GE激活AMPKα抑制心肌ROS的积累,从而阻断NLRP3炎性体介导的心肌细胞的凋亡和凋亡,并改善败血症小鼠的心脏功能。
更新日期:2020-02-20
中文翻译:

ip子苷可通过AMPKα依赖性途径来预防败血症引起的心肌功能障碍。
脓毒症引起的心肌功能异常与失控的炎症反应以及随后的心肌细胞丢失(凋亡和焦磷酸化)密切相关。我们以前的研究发现子苷(GE)可以保护鼠类心脏免于肥胖引起的炎症。然而,GE对败血症相关的心脏功能障碍的影响仍然未知。将小鼠暴露于脂多糖(LPS),以产生败血症诱导的心肌功能障碍。50 mg / kg GE连续7天用于治疗小鼠。我们的结果表明,GE的治疗显着提高了存活率和心脏功能,并抑制了LPS治疗小鼠的心肌炎症反应以及心肌损失。在那些NOD样受体蛋白3(NLRP3)缺陷小鼠中,GE的这些作用在很大程度上被消除了。进一步的检测表明,NLRP3炎性体激活的抑制取决于GE对p47phox的减少。GE处理恢复败血症小鼠心脏中AMP活化蛋白激酶α(AMPKα)的磷酸化和活性,敲除AMPKα取消了GE对败血症中活性氧(ROS)积累,NLRP3炎症小体活化和心肌细胞丢失的保护作用老鼠。总之,我们的发现表明,GE激活AMPKα抑制心肌ROS的积累,从而阻断NLRP3炎性体介导的心肌细胞的凋亡和凋亡,并改善败血症小鼠的心脏功能。GE处理恢复败血症小鼠心脏中AMP活化蛋白激酶α(AMPKα)的磷酸化和活性,敲除AMPKα取消了GE对败血症中活性氧(ROS)积累,NLRP3炎症小体活化和心肌细胞丢失的保护作用老鼠。总之,我们的发现表明,GE激活AMPKα抑制心肌ROS的积累,从而阻断NLRP3炎性体介导的心肌细胞的凋亡和凋亡,并改善败血症小鼠的心脏功能。GE处理恢复败血症小鼠心脏中AMP活化蛋白激酶α(AMPKα)的磷酸化和活性,敲除AMPKα取消了GE对败血症中活性氧(ROS)积累,NLRP3炎症小体活化和心肌细胞丢失的保护作用老鼠。总之,我们的发现表明,GE激活AMPKα抑制心肌ROS的积累,从而阻断NLRP3炎性体介导的心肌细胞的凋亡和凋亡,并改善败血症小鼠的心脏功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号