当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A low glycemic diet protects disease-prone Nrf2-deficient mice against age-related macular degeneration.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-02-14 , DOI: 10.1016/j.freeradbiomed.2020.02.010 Sheldon Rowan 1 , Shuhong Jiang 2 , Min-Lee Chang 3 , Jonathan Volkin 3 , Christa Cassalman 4 , Kelsey M Smith 5 , Matthew D Streeter 6 , David A Spiegel 6 , Carlos Moreira-Neto 7 , Naila Rabbani 8 , Paul J Thornalley 9 , Donald E Smith 3 , Nadia K Waheed 7 , Allen Taylor 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-02-14 , DOI: 10.1016/j.freeradbiomed.2020.02.010 Sheldon Rowan 1 , Shuhong Jiang 2 , Min-Lee Chang 3 , Jonathan Volkin 3 , Christa Cassalman 4 , Kelsey M Smith 5 , Matthew D Streeter 6 , David A Spiegel 6 , Carlos Moreira-Neto 7 , Naila Rabbani 8 , Paul J Thornalley 9 , Donald E Smith 3 , Nadia K Waheed 7 , Allen Taylor 1
Affiliation
|
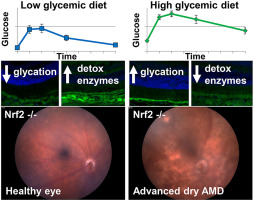
Age-related macular degeneration (AMD) is a major blinding disease, affecting over 14% of the elderly. Risk for AMD is related to age, diet, environment, and genetics. Dietary modulation of AMD risk is a promising treatment modality, but requires appropriate animal models to demonstrate advantages of diet. Mice lacking the antioxidant transcription factor Nrf2 (Nfe2l2) develop age-related retinopathy relevant to human AMD. Here we evaluated the effect of consuming high glycemic (HG) or low glycemic (LG) diets until 18-months of age on development of features relevant to AMD in Nrf2-null mice. Nrf2-null mice that consumed HG diets developed atrophic AMD, characterized by photoreceptor degeneration, retinal pigment epithelium (RPE) atrophy and pigmentary abnormalities, basal laminar deposits, and loss of the choriocapillaris. In contrast, Nrf2-null-mice that consumed LG diets did not develop retinal disease phenotypes. Consumption of HG diets was associated with accumulation of advanced glycation end-products in the RPE and systemically, whereas consumption of the LG diet was associated with increased levels of anti-glycative and anti-oxidative detoxification machinery. Together our data indicate that the Nrf2-null HG mouse is a good model for atrophic AMD studies and that the LG diet can activate protective pathways to prevent AMD, even in a genetically predisposed animal.
中文翻译:

低血糖饮食可保护易患疾病的 Nrf2 缺陷小鼠免受与年龄相关的黄斑变性。
年龄相关性黄斑变性 (AMD) 是一种主要的致盲疾病,影响超过 14% 的老年人。AMD 的风险与年龄、饮食、环境和遗传有关。AMD 风险的饮食调节是一种有前途的治疗方式,但需要适当的动物模型来证明饮食的优势。缺乏抗氧化转录因子 Nrf2 (Nfe2l2) 的小鼠会出现与人类 AMD 相关的年龄相关性视网膜病变。在这里,我们评估了在 18 个月大之前食用高血糖 (HG) 或低血糖 (LG) 饮食对 Nrf2 缺失小鼠中与 AMD 相关的特征发育的影响。食用 HG 饮食的 Nrf2 缺失小鼠发展为萎缩性 AMD,其特征是光感受器变性、视网膜色素上皮 (RPE) 萎缩和色素异常、基底层沉积和脉络膜毛细血管丧失。相比之下,食用 LG 饮食的 Nrf2 无效小鼠未出现视网膜疾病表型。食用 HG 饮食与 RPE 和全身晚期糖基化终产物的积累有关,而食用 LG 饮食与抗糖化和抗氧化解毒机制水平的增加有关。我们的数据共同表明,Nrf2-null HG 小鼠是萎缩性 AMD 研究的良好模型,并且 LG 饮食可以激活保护途径以预防 AMD,即使在遗传易感动物中也是如此。而食用 LG 饮食与抗糖化和抗氧化解毒机制水平的增加有关。我们的数据共同表明,Nrf2-null HG 小鼠是萎缩性 AMD 研究的良好模型,并且 LG 饮食可以激活保护途径以预防 AMD,即使在遗传易感动物中也是如此。而食用 LG 饮食与抗糖化和抗氧化解毒机制水平的增加有关。我们的数据共同表明,Nrf2-null HG 小鼠是萎缩性 AMD 研究的良好模型,并且 LG 饮食可以激活保护途径以预防 AMD,即使在遗传易感动物中也是如此。
更新日期:2020-02-20
中文翻译:

低血糖饮食可保护易患疾病的 Nrf2 缺陷小鼠免受与年龄相关的黄斑变性。
年龄相关性黄斑变性 (AMD) 是一种主要的致盲疾病,影响超过 14% 的老年人。AMD 的风险与年龄、饮食、环境和遗传有关。AMD 风险的饮食调节是一种有前途的治疗方式,但需要适当的动物模型来证明饮食的优势。缺乏抗氧化转录因子 Nrf2 (Nfe2l2) 的小鼠会出现与人类 AMD 相关的年龄相关性视网膜病变。在这里,我们评估了在 18 个月大之前食用高血糖 (HG) 或低血糖 (LG) 饮食对 Nrf2 缺失小鼠中与 AMD 相关的特征发育的影响。食用 HG 饮食的 Nrf2 缺失小鼠发展为萎缩性 AMD,其特征是光感受器变性、视网膜色素上皮 (RPE) 萎缩和色素异常、基底层沉积和脉络膜毛细血管丧失。相比之下,食用 LG 饮食的 Nrf2 无效小鼠未出现视网膜疾病表型。食用 HG 饮食与 RPE 和全身晚期糖基化终产物的积累有关,而食用 LG 饮食与抗糖化和抗氧化解毒机制水平的增加有关。我们的数据共同表明,Nrf2-null HG 小鼠是萎缩性 AMD 研究的良好模型,并且 LG 饮食可以激活保护途径以预防 AMD,即使在遗传易感动物中也是如此。而食用 LG 饮食与抗糖化和抗氧化解毒机制水平的增加有关。我们的数据共同表明,Nrf2-null HG 小鼠是萎缩性 AMD 研究的良好模型,并且 LG 饮食可以激活保护途径以预防 AMD,即使在遗传易感动物中也是如此。而食用 LG 饮食与抗糖化和抗氧化解毒机制水平的增加有关。我们的数据共同表明,Nrf2-null HG 小鼠是萎缩性 AMD 研究的良好模型,并且 LG 饮食可以激活保护途径以预防 AMD,即使在遗传易感动物中也是如此。











































 京公网安备 11010802027423号
京公网安备 11010802027423号