当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-directed mutagenesis of β sesquiphellandrene synthase enhances enzyme promiscuity
Phytochemistry ( IF 3.2 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.phytochem.2020.112286 De-Sheng Ker 1 , Kok Gan Chan 2 , Roohaida Othman 3 , Maizom Hassan 1 , Chyan Leong Ng 1
Phytochemistry ( IF 3.2 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.phytochem.2020.112286 De-Sheng Ker 1 , Kok Gan Chan 2 , Roohaida Othman 3 , Maizom Hassan 1 , Chyan Leong Ng 1
Affiliation
|
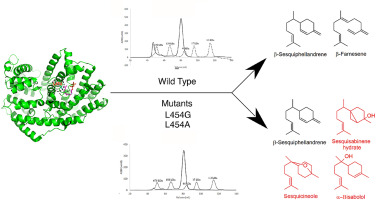
The chemical formation of terpenes in nature is carried out by terpene synthases as the main biocatalysts to guide the carbocation intermediate to form structurally diverse compounds including acyclic, mono- and multiple cyclic products. Despite intensive study of the enzyme active site, the mechanism of specific terpene biosynthesis remains unclear. Here we demonstrate that a single mutation of the amino acid L454G or L454A in the active site of Persicaria minor β-sesquiphellandrene synthase leads to a more promiscuous enzyme that is capable of producing additional hydroxylated sesquiterpenes such as sesquicineole, sesquisabinene hydrate and α-bisabolol. Furthermore, the same L454 residue mutation (L454G or L454A) in the active site also improves the protein homogeneity compared to the wild type protein. Taken together, our results demonstrate that residue Leucine 454 in the active site of β-sesquiphellandrene synthase is important for sesquiterpene product diversity as well as the protein homogeneity in solution.
中文翻译:

β倍半叶兰烯合酶的定点诱变增强酶的混杂性
萜烯在自然界中的化学形成是由萜烯合酶作为主要生物催化剂进行的,以引导碳阳离子中间体形成结构多样的化合物,包括无环、单环和多环产物。尽管对酶活性位点进行了深入研究,但特定萜烯生物合成的机制仍不清楚。在这里,我们证明了在 Persicaria minor β-倍半叶兰烯合酶的活性位点中氨基酸 L454G 或 L454A 的单个突变会导致更混杂的酶,该酶能够产生额外的羟基化倍半萜烯,例如倍半萜烯、倍半萜烯水合物和 α-没药醇。此外,与野生型蛋白质相比,活性位点中相同的 L454 残基突变(L454G 或 L454A)也提高了蛋白质的同质性。综合起来,
更新日期:2020-05-01
中文翻译:

β倍半叶兰烯合酶的定点诱变增强酶的混杂性
萜烯在自然界中的化学形成是由萜烯合酶作为主要生物催化剂进行的,以引导碳阳离子中间体形成结构多样的化合物,包括无环、单环和多环产物。尽管对酶活性位点进行了深入研究,但特定萜烯生物合成的机制仍不清楚。在这里,我们证明了在 Persicaria minor β-倍半叶兰烯合酶的活性位点中氨基酸 L454G 或 L454A 的单个突变会导致更混杂的酶,该酶能够产生额外的羟基化倍半萜烯,例如倍半萜烯、倍半萜烯水合物和 α-没药醇。此外,与野生型蛋白质相比,活性位点中相同的 L454 残基突变(L454G 或 L454A)也提高了蛋白质的同质性。综合起来,











































 京公网安备 11010802027423号
京公网安备 11010802027423号