Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ROR-α-1 inhibits the proliferation, invasion, and migration of hepatocellular carcinoma MHCC97H via downregulation of chemokine CXCL5
Cytokine ( IF 3.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.cyto.2020.155004 Gao Liu 1 , Zhang-Fu Yang 1 , Pei-Yun Zhou 1 , Cheng Zhou 1 , Ruo-Yu Guan 1 , Bao-Ye Sun 1 , Jia Fan 1 , Jian Zhou 1 , Yong Yi 1 , Shuang-Jian Qiu 1
Cytokine ( IF 3.7 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.cyto.2020.155004 Gao Liu 1 , Zhang-Fu Yang 1 , Pei-Yun Zhou 1 , Cheng Zhou 1 , Ruo-Yu Guan 1 , Bao-Ye Sun 1 , Jia Fan 1 , Jian Zhou 1 , Yong Yi 1 , Shuang-Jian Qiu 1
Affiliation
|
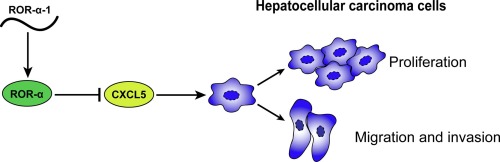
Hepatocarcinogenesis is a complicated process that is affected by a variety of microenvironmental factors, such as secretory chemokines and cell-extracellular matrix (ECM). Retinoic acid receptor-related orphan receptor (ROR)-α has been shown to attenuate tumor invasiveness by inducing suppressive cell microenvironment, and its low expression was associated with a worse prognosis in HCC patients. In the present study, we attempted to investigate the role and mechanism of the dominant transcript of ROR-α, ROR-α-1, in HCC development and progression. Among the four transcripts (ROR-α-1/-2/-3/-4), overexpression of ROR-α-1 dramatically suppressed the capacity of MHCC97H cells to proliferate, migrate and invade. We analyzed the differentially expressed genes in ROR-α-1-overexpressed and non-overexpressed MHCC97H cells, performed Gene Ontology (GO) enrichment analysis on these differentially-expressed genes, and found out that factors involved in the tumor microenvironment and ECM are related to the anti-tumor effects of ROR-α-1. Among these factors, chemokine CXCL5 was significantly downregulated by ROR-α-1 overexpression. Overexpression of ROR-α-1 remarkably inhibited the capacity of HCC cells to proliferate, migrate, invade, and downregulated the protein levels of β-catenin, c-Myc, Cyclin D1, and N-cadherin, suggesting the tumor-suppressive role of ROR-α-1 in MHCC97H cells. Moreover, overexpression of CXCL5 dramatically attenuated the suppressive effects of cell proliferation, migration and invasion induced by ROR-α-1 overexpression in MHCC97H, suggesting that ROR-α-1 exerts its anti-tumor effects via downregulating CXCL5. In conclusion, we demonstrate the tumor-suppressive role of ROR-α-1 in MHCC97H cells and that ROR-α-1 might play a tumor-suppressive role via regulation of chemokine CXCL5.
中文翻译:

ROR-α-1通过下调趋化因子CXCL5抑制肝细胞癌MHCC97H的增殖、侵袭和迁移
肝癌发生是一个复杂的过程,受多种微环境因素的影响,如分泌趋化因子和细胞外基质 (ECM)。视黄酸受体相关孤儿受体 (ROR)-α 已被证明通过诱导抑制性细胞微环境来减弱肿瘤侵袭性,其低表达与 HCC 患者的较差预后相关。在本研究中,我们试图研究 ROR-α 的主要转录物 ROR-α-1 在 HCC 发展和进展中的作用和机制。在四种转录本(ROR-α-1/-2/-3/-4)中,ROR-α-1的过表达显着抑制了MHCC97H细胞增殖、迁移和侵袭的能力。我们分析了 ROR-α-1 过表达和非过表达 MHCC97H 细胞中差异表达的基因,对这些差异表达基因进行基因本体(GO)富集分析,发现肿瘤微环境和ECM相关因素与ROR-α-1的抗肿瘤作用有关。在这些因素中,趋化因子 CXCL5 被 ROR-α-1 过表达显着下调。ROR-α-1 的过表达显着抑制 HCC 细胞增殖、迁移、侵袭的能力,并下调 β-catenin、c-Myc、Cyclin D1 和 N-cadherin 的蛋白质水平,表明 ROR-α-1 的肿瘤抑制作用MHCC97H 细胞中的 ROR-α-1。此外,CXCL5的过表达显着减弱了MHCC97H中ROR-α-1过表达诱导的细胞增殖、迁移和侵袭的抑制作用,表明ROR-α-1通过下调CXCL5发挥其抗肿瘤作用。综上所述,
更新日期:2020-05-01
中文翻译:

ROR-α-1通过下调趋化因子CXCL5抑制肝细胞癌MHCC97H的增殖、侵袭和迁移
肝癌发生是一个复杂的过程,受多种微环境因素的影响,如分泌趋化因子和细胞外基质 (ECM)。视黄酸受体相关孤儿受体 (ROR)-α 已被证明通过诱导抑制性细胞微环境来减弱肿瘤侵袭性,其低表达与 HCC 患者的较差预后相关。在本研究中,我们试图研究 ROR-α 的主要转录物 ROR-α-1 在 HCC 发展和进展中的作用和机制。在四种转录本(ROR-α-1/-2/-3/-4)中,ROR-α-1的过表达显着抑制了MHCC97H细胞增殖、迁移和侵袭的能力。我们分析了 ROR-α-1 过表达和非过表达 MHCC97H 细胞中差异表达的基因,对这些差异表达基因进行基因本体(GO)富集分析,发现肿瘤微环境和ECM相关因素与ROR-α-1的抗肿瘤作用有关。在这些因素中,趋化因子 CXCL5 被 ROR-α-1 过表达显着下调。ROR-α-1 的过表达显着抑制 HCC 细胞增殖、迁移、侵袭的能力,并下调 β-catenin、c-Myc、Cyclin D1 和 N-cadherin 的蛋白质水平,表明 ROR-α-1 的肿瘤抑制作用MHCC97H 细胞中的 ROR-α-1。此外,CXCL5的过表达显着减弱了MHCC97H中ROR-α-1过表达诱导的细胞增殖、迁移和侵袭的抑制作用,表明ROR-α-1通过下调CXCL5发挥其抗肿瘤作用。综上所述,











































 京公网安备 11010802027423号
京公网安备 11010802027423号