当前位置:
X-MOL 学术
›
Cell Calcium
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-channel properties of skeletal muscle ryanodine receptor pore Δ4923FF4924 in two brothers with a lethal form of fetal akinesia.
Cell Calcium ( IF 4.3 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.ceca.2020.102182 Le Xu 1 , Frederike L Harms 2 , Venkat R Chirasani 3 , Daniel A Pasek 1 , Fanny Kortüm 2 , Peter Meinecke 2 , Nikolay V Dokholyan 3 , Kerstin Kutsche 2 , Gerhard Meissner 1
Cell Calcium ( IF 4.3 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.ceca.2020.102182 Le Xu 1 , Frederike L Harms 2 , Venkat R Chirasani 3 , Daniel A Pasek 1 , Fanny Kortüm 2 , Peter Meinecke 2 , Nikolay V Dokholyan 3 , Kerstin Kutsche 2 , Gerhard Meissner 1
Affiliation
|
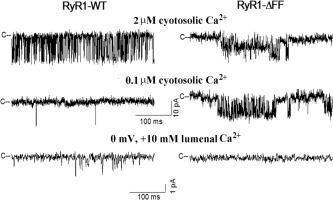
Ryanodine receptor ion channels (RyR1s) release Ca2+ ions from the sarcoplasmic reticulum to regulate skeletal muscle contraction. By whole-exome sequencing, we identified the heterozygous RYR1 variant c.14767_14772del resulting in the in-frame deletion p.(Phe4923_Phe4924del) in two brothers with a lethal form of the fetal akinesia deformation syndrome (FADS). The two deleted phenylalanines (RyR1-Δ4923FF4924) are located in the S6 pore-lining helix of RyR1. Clinical features in one of the two siblings included severe hypotonia, thin ribs, swallowing inability, and respiratory insufficiency that caused early death. Functional consequences of the RyR1-Δ4923FF4924 variant were determined using recombinant 2,200-kDa homotetrameric and heterotetrameric RyR1 channel complexes that were expressed in HEK293 cells and characterized by cellular, electrophysiological, and computational methods. Cellular Ca2+ release in response to caffeine indicated that the homotetrameric variant formed caffeine-sensitive Ca2+ conducting channels in HEK293 cells. In contrast, the homotetrameric channel complex was not activated by Ca2+ and did not conduct Ca2+ based on single-channel measurements. The computational analysis suggested decreased protein stability and loss of salt bridge interactions between RyR1-R4944 and RyR1-D4938, increasing the electrostatic interaction energy of Ca2+ in a region 20 Å from the mutant site. Co-expression of wild-type and mutant RyR1s resulted in Ca2+-dependent channel activities that displayed intermediate Ca2+ conductances and suggested maintenance of a reduced Ca2+ release in the two patients. Our findings reveal that the RYR1 pore variant p.(Phe4923_Phe4924del) attenuates the flow of Ca2+ through heterotetrameric channels, but alone was not sufficient to cause FADS, indicating additional genetic factors to be involved.
中文翻译:

具有致命形式的胎儿运动障碍的两个兄弟的骨骼肌ryanodine受体孔Δ4923FF4924的单通道特性。
Ryanodine受体离子通道(RyR1s)从肌质网释放Ca2 +离子,以调节骨骼肌收缩。通过全外显子测序,我们鉴定出杂合的RYR1变体c.14767_14772del,导致两个兄弟中的帧内缺失p。(Phe4923_Phe4924del),具有致命的胎儿运动能力变形综合征(FADS)。两个缺失的苯丙氨酸(RyR1-Δ4923FF4924)位于RyR1的S6孔衬螺旋中。这两个兄弟姐妹之一的临床特征包括严重的肌张力低下,肋骨薄,吞咽能力不足和呼吸功能不全而导致早期死亡。RyR1-Δ4923FF4924变体的功能后果是通过在HEK293细胞中表达并通过细胞,电生理学和计算方法。响应咖啡因的细胞Ca2 +释放表明同四聚体变体在HEK293细胞中形成了咖啡因敏感的Ca2 +传导通道。相反,基于单通道测量,同四聚体通道复合物未被Ca2 +激活,并且不传导Ca2 +。计算分析表明,蛋白质稳定性降低,RyR1-R4944和RyR1-D4938之间的盐桥相互作用丧失,从而增加了距突变位点20Å处Ca2 +的静电相互作用能。野生型和突变型RyR1的共表达导致依赖Ca2 +的通道活动,显示出中等的Ca2 +电导率,并建议维持这两名患者的Ca2 +释放减少。我们的发现揭示了RYR1孔变体p。
更新日期:2020-02-17
中文翻译:

具有致命形式的胎儿运动障碍的两个兄弟的骨骼肌ryanodine受体孔Δ4923FF4924的单通道特性。
Ryanodine受体离子通道(RyR1s)从肌质网释放Ca2 +离子,以调节骨骼肌收缩。通过全外显子测序,我们鉴定出杂合的RYR1变体c.14767_14772del,导致两个兄弟中的帧内缺失p。(Phe4923_Phe4924del),具有致命的胎儿运动能力变形综合征(FADS)。两个缺失的苯丙氨酸(RyR1-Δ4923FF4924)位于RyR1的S6孔衬螺旋中。这两个兄弟姐妹之一的临床特征包括严重的肌张力低下,肋骨薄,吞咽能力不足和呼吸功能不全而导致早期死亡。RyR1-Δ4923FF4924变体的功能后果是通过在HEK293细胞中表达并通过细胞,电生理学和计算方法。响应咖啡因的细胞Ca2 +释放表明同四聚体变体在HEK293细胞中形成了咖啡因敏感的Ca2 +传导通道。相反,基于单通道测量,同四聚体通道复合物未被Ca2 +激活,并且不传导Ca2 +。计算分析表明,蛋白质稳定性降低,RyR1-R4944和RyR1-D4938之间的盐桥相互作用丧失,从而增加了距突变位点20Å处Ca2 +的静电相互作用能。野生型和突变型RyR1的共表达导致依赖Ca2 +的通道活动,显示出中等的Ca2 +电导率,并建议维持这两名患者的Ca2 +释放减少。我们的发现揭示了RYR1孔变体p。











































 京公网安备 11010802027423号
京公网安备 11010802027423号