Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Autophagic Degradation of NBR1 Restricts Metastatic Outgrowth during Mammary Tumor Progression.
Developmental Cell ( IF 10.7 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.devcel.2020.01.025 Timothy Marsh 1 , Candia M Kenific 1 , Deepthisri Suresh 2 , Hugo Gonzalez 3 , Eliah R Shamir 2 , Wenbin Mei 3 , Alexandra Tankka 2 , Andrew M Leidal 2 , Sandhya Kalavacherla 3 , Kimberly Woo 2 , Zena Werb 4 , Jayanta Debnath 5
Developmental Cell ( IF 10.7 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.devcel.2020.01.025 Timothy Marsh 1 , Candia M Kenific 1 , Deepthisri Suresh 2 , Hugo Gonzalez 3 , Eliah R Shamir 2 , Wenbin Mei 3 , Alexandra Tankka 2 , Andrew M Leidal 2 , Sandhya Kalavacherla 3 , Kimberly Woo 2 , Zena Werb 4 , Jayanta Debnath 5
Affiliation
|
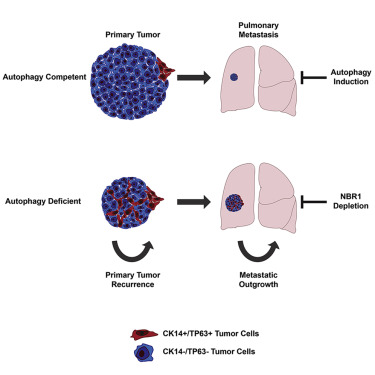
Although autophagy is being pursued as a therapeutic target in clinical oncology trials, its effects on metastasis, the principal cause of cancer mortality, remain unclear. Here, we utilize mammary cancer models to temporally delete essential autophagy regulators during carcinoma progression. Though genetic ablation of autophagy strongly attenuates primary mammary tumor growth, impaired autophagy promotes spontaneous metastasis and enables the outgrowth of disseminated tumor cells into overt macro-metastases. Transcriptomic analysis reveals that autophagy deficiency elicits a subpopulation of otherwise luminal tumor cells exhibiting basal differentiation traits, which is reversed upon preventing accumulation of the autophagy cargo receptor, Neighbor to BRCA1 (NBR1). Furthermore, pharmacological and genetic induction of autophagy suppresses pro-metastatic differentiation and metastatic outgrowth. Analysis of human breast cancer data reveal that autophagy gene expression inversely correlates with pro-metastatic differentiation signatures and predicts overall and distant metastasis-free survival. Overall, these findings highlight autophagy-dependent control of NBR1 as a key determinant of metastatic progression.
中文翻译:

NBR1的自噬降解限制了乳腺肿瘤进展过程中的转移性生长。
尽管自噬在临床肿瘤学试验中被视为治疗靶标,但其对转移的影响尚不清楚,转移是癌症死亡的主要原因。在这里,我们利用乳腺癌模型在癌症进展过程中暂时删除必需的自噬调节剂。尽管自噬的基因消融强烈地减弱了原发性乳腺肿瘤的生长,但是自噬受损会促进自发转移,并使已扩散的肿瘤细胞向过度的大转移转移。转录组学分析表明,自噬缺陷会引起其他具有基础分化特征的腔肿瘤细胞亚群,一旦阻止自噬货物受体(BRCA1,NBR1)的积累,这一现象就会逆转。此外,自噬的药理和遗传诱导抑制促转移分化和转移性生长。对人类乳腺癌数据的分析表明,自噬基因表达与促转移分化特征成反比,并预测总体和远处无转移生存。总体而言,这些发现强调了NBR1的自噬依赖性控制是转移进展的关键决定因素。
更新日期:2020-02-20
中文翻译:

NBR1的自噬降解限制了乳腺肿瘤进展过程中的转移性生长。
尽管自噬在临床肿瘤学试验中被视为治疗靶标,但其对转移的影响尚不清楚,转移是癌症死亡的主要原因。在这里,我们利用乳腺癌模型在癌症进展过程中暂时删除必需的自噬调节剂。尽管自噬的基因消融强烈地减弱了原发性乳腺肿瘤的生长,但是自噬受损会促进自发转移,并使已扩散的肿瘤细胞向过度的大转移转移。转录组学分析表明,自噬缺陷会引起其他具有基础分化特征的腔肿瘤细胞亚群,一旦阻止自噬货物受体(BRCA1,NBR1)的积累,这一现象就会逆转。此外,自噬的药理和遗传诱导抑制促转移分化和转移性生长。对人类乳腺癌数据的分析表明,自噬基因表达与促转移分化特征成反比,并预测总体和远处无转移生存。总体而言,这些发现强调了NBR1的自噬依赖性控制是转移进展的关键决定因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号