Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.mcat.2020.110819 Thiago de Sousa Fonseca , Kimberly Benedetti Vega , Marcos Reinaldo da Silva , Maria da Conceição Ferreira de Oliveira , Telma Leda Gomes de Lemos , Martina Letizia Contente , Francesco Molinari , Marco Cespugli , Sara Fortuna , Lucia Gardossi , Marcos Carlos de Mattos
|
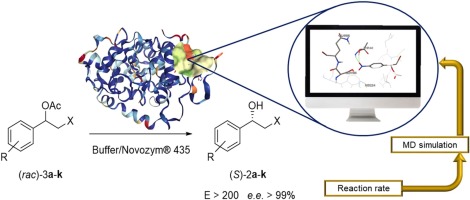
Racemic phenylethyl halohydrins acetates containing several groups attached to the aromatic ring were resolved via hydrolysis reaction in the presence of lipase B from Candida antarctica (Novozym® 435). In all cases, the kinetic resolution was highly selective (E > 200) leading to the corresponding (S)-β-halohydrin with ee > 99 %. However, the time required for an ideal 50 % conversion ranged from 15 min for 2,4-dichlorophenyl chlorohydrin acetate to 216 h for 2-chlorophenyl bromohydrin acetate. Six chlorohydrins and five bromohydrins were evaluated, the latter being less reactive. For the β-brominated substrates, steric hindrance on the aromatic ring played a crucial role, which was not observed for the β-chlorinated derivatives. To shed light on the different reaction rates, docking studies were carried out with all the substrates using MD simulations. The computational data obtained for the β-brominated substrates, based on the parameters analysed such as NAC (near attack conformation), distance between Ser-O and carbonyl-C and oxyanion site stabilization were in agreement with the experimental results. On the other hand, the data obtained for β-chlorinated substrates suggested that physical aspects such as high hydrophobicity or induced change in the conformation of the enzymatic active site are more relevant aspects when compared to steric hindrance effects.
中文翻译:

脂肪酶介导的苯乙基卤代醇乙酸盐的酶促动力学拆分:研究与合理化案例
在来自南极假丝酵母的脂肪酶B (435)的存在下,通过水解反应将含有与芳香环相连的多个基团的外消旋苯乙基卤代醇乙酸酯分解。在所有情况下,动力学拆分都是高度选择性的(E> 200),导致相应的拆分(See> 99%的)-β-卤代醇。但是,理想的50%转化所需的时间为2,4-二氯苯基氯醇乙酸盐的15分钟到2-氯苯基溴醇乙酸盐的216小时。评价了六种氯醇和五种溴醇,后者的反应性较低。对于β-溴化的底物,芳香环上的位阻起着至关重要的作用,而对于β-氯代衍生物则没有观察到。为了阐明不同的反应速率,使用MD模拟对所有底物进行了对接研究。基于分析的参数(例如NAC(近攻构象),Ser-O与羰基-C之间的距离和氧阴离子位点稳定化)获得的有关β-溴化底物的计算数据与实验结果相符。另一方面,











































 京公网安备 11010802027423号
京公网安备 11010802027423号