Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.jcat.2020.01.027 Minkyu Kim , Austin D. Franklin , Rachel Martin , Yingxue Bian , Jason F. Weaver , Aravind Asthagiri
|
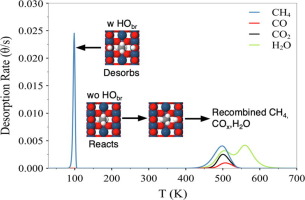
The ability of the IrO2(1 1 0) surface to promote CH4 activation at low temperatures (~150 K) suggests possibilities for developing IrO2-based catalysts to selectively convert light alkanes to high-value chemicals. In this study, we present experimental results and microkinetic simulations, based on density functional theory (DFT) calculations, of temperature programmed reaction spectra (TPRS) obtained for CH4 σ-complexes adsorbed on IrO2(1 1 0), and focus on clarifying how surface OH groups modify the branching between CH4 desorption, activation and subsequent reactions during TPRS. Our DFT results predict that surface OH groups strongly destabilize CH4 σ-complexes on IrO2(1 1 0) in addition to deactivating surface O-atoms that are needed to achieve CH4 activation at low temperature. We demonstrate that a microkinetic model that incorporates the influence of surface OH groups on the CH4 binding and reactivity reproduces experimental TPRS results which show that adsorbed CH4 σ-complexes preferentially dissociate at low CH4 coverage, but that an increasing fraction of the adsorbed CH4 desorbs at low temperature (~120 K) with increasing initial CH4 or H coverage. The simulations reveal that low-temperature CH4 desorption during TPRS arises primarily from CH4 σ-complexes that are kinetically-trapped between adjacent surface OH groups, and are thus destabilized and unable to access reactive O-atoms. In addition, when the effect of adjacent OH groups is incorporated we find that the PBE-D3 functional incorporating dispersion provides CH4 binding energies that agree more closely with that obtained from the TPRS experiments. Our results demonstrate that the local effect of adjacent OH groups must be incorporated into any microkinetic models to properly capture the selectivity between extensive and partial oxidation in alkane conversion on IrO2(1 1 0) under reaction conditions.
中文翻译:

IrO 2(1 1 0)上低温甲烷活化的动力学:局部表面氢氧化物的作用
IrO 2(1 1 0)表面在低温(〜150 K)下促进CH 4活化的能力表明,开发基于IrO 2的催化剂将轻质烷烃选择性转化为高价值化学品的可能性。在这项研究中,我们目前的实验结果和microkinetic模拟,基于密度泛函理论的CH得到(DFT)计算,程序升温反应谱(的TPR)4吸附在的IrOσ络合物2(110),并集中在阐明表面OH基团如何修饰TPRS期间CH 4解吸,活化和后续反应之间的分支。我们的DFT结果预测表面OH基团会严重破坏CH 4的稳定性除了钝化在低温下实现CH 4活化所需的表面O原子外,还具有IrO 2(1 1 0)上的σ络合物。我们表明,并入在CH表面OH基团的影响的microkinetic模型4结合和反应性再现实验的TPR结果,其表明,吸附的CH 4 σ络合物在低温CH优先解离4覆盖,但该吸附的增加分数随着初始CH 4或H覆盖率的增加,CH 4在低温(〜120 K)下解吸。模拟表明,TPRS期间低温CH 4的解吸主要来自CH 4σ络合物被动力学俘获在相邻的表面OH基团之间,因此不稳定并且无法接近反应性O原子。另外,当掺入相邻的OH基团的作用时,我们发现掺入PBE-D3官能团的分散体提供的CH 4结合能与从TPRS实验获得的能更紧密地吻合。我们的结果表明,相邻的OH基团的局部作用必须纳入任何微动力学模型中,以在反应条件下正确捕获IrO 2(1 1 0)上烷烃转化中广泛氧化和部分氧化之间的选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号