Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.jcat.2020.01.012 Lihan Zhu , Haiyan Yuan , Jingping Zhang
|
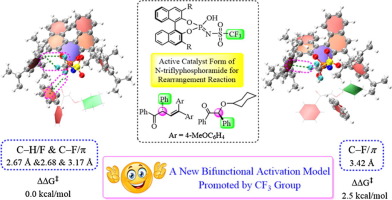
The acidity of the chiral Brønsted acid catalysts is crucial for the catalytic efficiency, the highly acidic BINOL-derived chiral N-triflylphosphoramides can provide effective asymmetric catalysis for rearrangement reactions. Despite multiple interactions between the basic/acidic sites of the functional group and the substrates have been evaluated, the origin of high catalytic activity and excellent enantioselelctivity of N-triflylphosphoramides catalyst is still challenging. Especially the role of CF3 groups in controlling activation mode and determining enantioselectivity. Thus, two effective case reactions of pinacol and acyloin rearrangement catalyzed by BINOL N-triflyphosphoramide were employed to provide basis understanding for these issues. Our calculations reveal that the P(=NTf)OH tautomer of N-triflyphosphoramide is an active catalyst form for the rearrangement reaction, differing from previous proposed model involving P(=O)NHTf group. This can be ascribed to the additional C–F···π interaction between the CF3 group of catalyst and the migration group of substrate in the preferred activation model. Furthermore, we found that the CF3 substituent on the central functional group effectively aids the bifunctional activation and improves catalytic activity of N-triflyphosphoramide catalyst. More importantly, the CF3 substituent and the orientation of migration groups play a significant role in controlling the enantioselectivity by contributing different strength multiple C–H···F, C–F···π, and C–H···π interactions between catalyst and substrate. Overall, our findings on the factors affecting the stereochemical control may show utility for other challenging asymmetric reactions catalyzed by chiral N-triflylphosphoramides.
中文翻译:

CF 3官能团取代基对双功能激活模型和BINOL N-三氟磷酰胺催化重排反应的对映选择性的影响
手性布朗斯台德酸催化剂的酸度对于催化效率至关重要,高酸性的BINOL衍生的手性N-三氟磷酰胺可为重排反应提供有效的不对称催化。尽管已经评估了官能团的碱性/酸性位点与底物之间的多重相互作用,但是N-三氟磷酰胺催化剂的高催化活性和出色的对映选择性的起源仍然具有挑战性。特别是CF 3的作用在控制激活模式和确定对映选择性中进行分组。因此,采用了由宾诺尔N-三氟代磷酰胺催化的频哪醇和酰基肌醇重排的两个有效案例反应,为对这些问题的理解提供了基础。我们的计算表明,N-三氟磷酰胺的P(= NTf)OH互变异构体是重排反应的活性催化剂形式,与先前提出的涉及P(= O)NHTf基团的模型不同。这可以归因于附加CF ... π的CF之间的相互作用3组催化剂的,并在优选的活化模型中的迁移组的衬底。此外,我们发现CF 3中心官能团上的取代基有效地帮助双功能活化并改善了N-三氟磷酰胺催化剂的催化活性。更重要的是,CF 3取代基和迁移基团的取向在控制对映选择性方面起着重要作用,它通过贡献不同强度的多个C–H···F,C–F··· π和C–H··· π催化剂和底物之间的相互作用。总的来说,我们对影响立体化学控制的因素的研究结果可能表明可用于手性N-三氟磷酸酰胺催化的其他挑战性不对称反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号