当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted melanoma radiotherapy using ultrasmall 177Lu-labeled α-melanocyte stimulating hormone-functionalized core-shell silica nanoparticles.
Biomaterials ( IF 12.8 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.biomaterials.2020.119858 Xiuli Zhang 1 , Feng Chen 2 , Melik Z Turker 3 , Kai Ma 4 , Pat Zanzonico 5 , Fabio Gallazzi 6 , Manankumar A Shah 1 , Austin R Prater 1 , Ulrich Wiesner 3 , Michelle S Bradbury 7 , Michael R McDevitt 2 , Thomas P Quinn 1
Biomaterials ( IF 12.8 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.biomaterials.2020.119858 Xiuli Zhang 1 , Feng Chen 2 , Melik Z Turker 3 , Kai Ma 4 , Pat Zanzonico 5 , Fabio Gallazzi 6 , Manankumar A Shah 1 , Austin R Prater 1 , Ulrich Wiesner 3 , Michelle S Bradbury 7 , Michael R McDevitt 2 , Thomas P Quinn 1
Affiliation
|
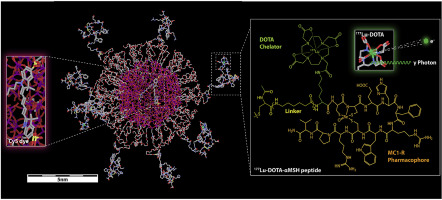
Lutetium-177 (177Lu) radiolabeled ultrasmall (~6 nm dia.) fluorescent core-shell silica nanoparticles (Cornell prime dots or C' dots) were developed for improving efficacy of targeted radiotherapy in melanoma models. PEGylated C' dots were surface engineered to display 10-15 alpha melanocyte stimulating hormone (αMSH) cyclic peptide analogs for targeting the melanocortin-1 receptor (MC1-R) over-expressed on melanoma tumor cells. The 177Lu-DOTA-αMSH-PEG-C' dot product was radiochemically stable, biologically active, and exhibited high affinity cellular binding properties and internalization. Selective tumor uptake and favorable biodistribution properties were also demonstrated, in addition to bulk renal clearance, in syngeneic B16F10 and human M21 xenografted models. Prolonged survival was observed in the treated cohorts relative to controls. Dosimetric analysis showed no excessively high absorbed dose among normal organs. Correlative histopathology of ex vivo treated tumor specimens revealed expected necrotic changes; no acute pathologic findings were noted in the liver or kidneys. Collectively, these results demonstrated that 177Lu-DOTA-αMSH-PEG-C' dot targeted melanoma therapy overcame the unfavorable biological properties and dose-limiting toxicities associated with existing mono-molecular treatments. The unique and tunable surface chemistries of this targeted ultrasmall radiotherapeutic, coupled with its favorable pharmacokinetic properties, substantially improved treatment efficacy and demonstrated a clear survival benefit in melanoma models, which supports its further clinical translation.
中文翻译:

使用超小型177Lu标记的α-黑素细胞刺激激素功能化的核壳二氧化硅纳米粒子进行靶向黑素瘤放疗。
开发了ute 177(177Lu)放射性标记的超小(直径约6 nm)荧光核-壳二氧化硅纳米粒子(康奈尔素点或C'点),以提高黑色素瘤模型中靶向放射治疗的功效。对聚乙二醇化的C'点进行表面处理,以显示10-15个α黑色素细胞刺激激素(αMSH)环状肽类似物,以靶向在黑色素瘤肿瘤细胞上过表达的melanocortin-1受体(MC1-R)。177Lu-DOTA-αMSH-PEG-C'点产物具有放射化学稳定性,生物活性,并具有高亲和力的细胞结合特性和内在化作用。在同种B16F10和人类M21异种移植模型中,除大量肾脏清除作用外,还证明了选择性肿瘤吸收和良好的生物分布特性。相对于对照,在治疗的人群中观察到延长的生存期。剂量学分析表明正常器官中没有吸收过高的剂量。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗效果,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。剂量学分析表明正常器官中没有吸收过高的剂量。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗效果,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。剂量学分析表明正常器官中没有吸收过高的剂量。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。
更新日期:2020-02-20
中文翻译:

使用超小型177Lu标记的α-黑素细胞刺激激素功能化的核壳二氧化硅纳米粒子进行靶向黑素瘤放疗。
开发了ute 177(177Lu)放射性标记的超小(直径约6 nm)荧光核-壳二氧化硅纳米粒子(康奈尔素点或C'点),以提高黑色素瘤模型中靶向放射治疗的功效。对聚乙二醇化的C'点进行表面处理,以显示10-15个α黑色素细胞刺激激素(αMSH)环状肽类似物,以靶向在黑色素瘤肿瘤细胞上过表达的melanocortin-1受体(MC1-R)。177Lu-DOTA-αMSH-PEG-C'点产物具有放射化学稳定性,生物活性,并具有高亲和力的细胞结合特性和内在化作用。在同种B16F10和人类M21异种移植模型中,除大量肾脏清除作用外,还证明了选择性肿瘤吸收和良好的生物分布特性。相对于对照,在治疗的人群中观察到延长的生存期。剂量学分析表明正常器官中没有吸收过高的剂量。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗效果,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。剂量学分析表明正常器官中没有吸收过高的剂量。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗效果,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。剂量学分析表明正常器官中没有吸收过高的剂量。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。离体治疗的肿瘤标本的相关组织病理学显示预期的坏死性改变。在肝脏或肾脏中未发现急性病理结果。总体而言,这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。这些结果表明,177Lu-DOTA-αMSH-PEG-C'点靶向黑色素瘤疗法克服了与现有单分子疗法相关的不利生物学特性和剂量限制性毒性。该靶向超小型放射治疗剂的独特且可调节的表面化学,加上其良好的药代动力学特性,大大改善了治疗功效,并在黑色素瘤模型中显示出明显的生存获益,这为其进一步的临床翻译提供了支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号