当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile Multivalent Redox Chemistries in Water-in-Bisalt Hydrogel Electrolytes for Hybrid Energy Storage Full Cells
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-02-20 , DOI: 10.1021/acsenergylett.0c00059 Jae Min Park 1 , Milan Jana 1 , Puritut Nakhanivej 1 , Bo-Kyong Kim 2 , Ho Seok Park 1, 3, 4
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-02-20 , DOI: 10.1021/acsenergylett.0c00059 Jae Min Park 1 , Milan Jana 1 , Puritut Nakhanivej 1 , Bo-Kyong Kim 2 , Ho Seok Park 1, 3, 4
Affiliation
|
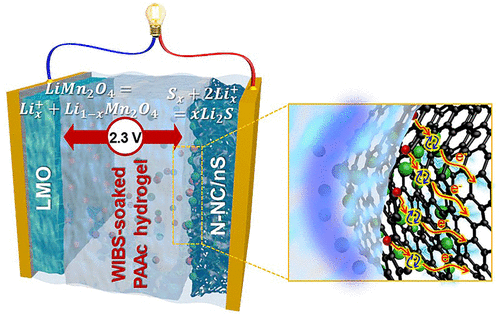
High-capacity electrode materials have been investigated to overcome the low energy density of electrochemical capacitors, but there are still issues arising from the trade-off between charge storage capacity and kinetics, efficiency, and stability. Herein, we describe multivalent sulfur redox chemistry for the high power and energy efficiency of hybrid energy storage full cells, where nitrogen-incorporated nanoporous carbon/nanosulfur (N-NC/nS) and lithium manganese oxide are configured into negative and positive electrodes, respectively, using water-in-bisalt (WIBS)-soaked poly(acrylic acid) hydrogel electrolyte. As confirmed by the major contribution of surface redox capacity to the total capacity, low activation energy, high exchange current density, and fast charge transfer, the N-NC/nS achieves facile surface redox kinetics arising from the hierarchical porosity, nanoscale confinement of nS, and high ionic conductivity of WIBS hydrogels. The resulting full cells deliver capacitor-like high power density of 15.7 kW kg–1, along with an energy density of 30.1 Wh kg–1, 78.7% retention over 2000 cycles, and an energy efficiency of 98%.
中文翻译:

玄武岩水包水凝胶电解质中用于混合储能全电池的简便多价氧化还原化学
为了克服电化学电容器的低能量密度,已经研究了高容量的电极材料,但是仍然存在由电荷存储容量与动力学,效率和稳定性之间的权衡所引起的问题。在这里,我们描述了混合能源储能全电池的高功率和高能效的多价硫氧化还原化学方法,其中将掺氮的纳米多孔碳/纳米硫(N-NC / nS)和锰酸锂分别配置为负极和正极,使用双盐包水(WIBS)浸泡的聚(丙烯酸)水凝胶电解质。如表面氧化还原容量对总容量,低活化能,高交换电流密度和快速电荷转移的主要贡献所证实,N-NC / nS实现了便捷的表面氧化还原动力学,这归因于WIBS水凝胶的分层孔隙度,nS的纳米级限制和高离子电导率。由此产生的全电池可提供类似电容器的15.7 kW kg高功率密度–1以及30.1 Wh kg kg -1的能量密度–在2000个循环中保留78.7%的能量,而能源效率为98%。
更新日期:2020-02-20
中文翻译:

玄武岩水包水凝胶电解质中用于混合储能全电池的简便多价氧化还原化学
为了克服电化学电容器的低能量密度,已经研究了高容量的电极材料,但是仍然存在由电荷存储容量与动力学,效率和稳定性之间的权衡所引起的问题。在这里,我们描述了混合能源储能全电池的高功率和高能效的多价硫氧化还原化学方法,其中将掺氮的纳米多孔碳/纳米硫(N-NC / nS)和锰酸锂分别配置为负极和正极,使用双盐包水(WIBS)浸泡的聚(丙烯酸)水凝胶电解质。如表面氧化还原容量对总容量,低活化能,高交换电流密度和快速电荷转移的主要贡献所证实,N-NC / nS实现了便捷的表面氧化还原动力学,这归因于WIBS水凝胶的分层孔隙度,nS的纳米级限制和高离子电导率。由此产生的全电池可提供类似电容器的15.7 kW kg高功率密度–1以及30.1 Wh kg kg -1的能量密度–在2000个循环中保留78.7%的能量,而能源效率为98%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号