当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidation of Cymantrene-Tagged Tamoxifen Analogues: Effect of Diphenyl Functionalization on the Redox Mechanism
Organometallics ( IF 2.5 ) Pub Date : 2020-02-20 , DOI: 10.1021/acs.organomet.9b00822 Kan Wu 1 , Bimal Pudasaini 2, 3 , Ji Young Park 2, 3 , Siden Top 4 , Gérard Jaouen 4, 5 , Mu-Hyun Baik 2, 3 , William E. Geiger 1
Organometallics ( IF 2.5 ) Pub Date : 2020-02-20 , DOI: 10.1021/acs.organomet.9b00822 Kan Wu 1 , Bimal Pudasaini 2, 3 , Ji Young Park 2, 3 , Siden Top 4 , Gérard Jaouen 4, 5 , Mu-Hyun Baik 2, 3 , William E. Geiger 1
Affiliation
|
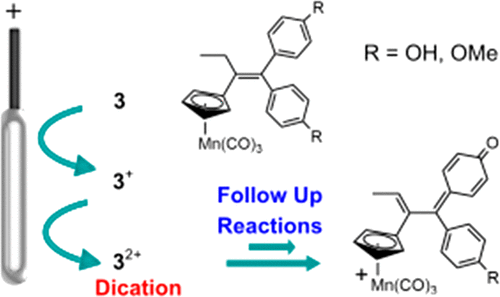
The oxidations of 1,1′-di-p-anisolyl-2-cymantrenylbutene (3b) and 1,1′-di-p-hydroxyphenyl-2-cymantrenylbutene (3c) were investigated by electrochemical and spectroscopic experiments and by density functional theory (DFT) calculations. Both compounds undergo a reversible one-electron oxidation followed closely by a partially chemically reversible second oxidation (E1/2 values vs ferrocene: 0.60 and 0.74 V for 3b; 0.63 and 0.78 V for 3c). In comparison to the nonphenyl-functionalized parent, 1,1′-diphenyl-2-cymantrenylbutene (3a), 3b,c have lower and more closely spaced oxidation potentials and more rapid follow-up reactions of their dications, 3b2+ and 3c2+. Shifts in the calculated charge distributions of the neutral compounds and their singly and doubly oxidized products corroborated trends in the measured shifts of Mn–CO νCO frequencies in assigning the redox sites primarily to the diarylbutene fragment. Upon removal of electrons, the lost charge density is partially compensated by the polarizable cymantrenyl tag. The half-lives of the dications 3b2+ and 3c2+ are about 10 s at room temperature in dichloromethane/0.05 M [NBu4][B(C6F5)4]. Their follow-up reactions are initiated by loss of a proton either from a hydroxyl group or from the CH2 group of the diarylbutene unit, giving rise to two products having quinone methide structures. Although the initial oxidation sites of cymantrene-tagged diarylbutenes are primarily ligand based and those of ferrocene-tagged diarylbutenes are metal-based, the ultimate oxidation products of their p-OH- or p-OMe-functionalized derivatives are very similar.
中文翻译:

Cymantrene标记的他莫昔芬类似物的氧化:联苯功能化对氧化还原机制的影响。
1,1'-二-的氧化p -anisolyl -2- cymantrenylbutene(图3b)和1,1'-二- p羟基苯基-2- cymantrenylbutene(3C)通过电化学和光谱实验和通过密度泛函理论研究(DFT)计算。两种化合物均进行可逆的单电子氧化,然后进行部分化学可逆的第二次氧化(相对于二茂铁,E 1/2值:3b为0.60和0.74 V ;3c为0.63和0.78 V )。与非苯基官能化母体相比,1,1'-二苯基-2-环丁烯基丁烯(3a),3b,c具有更低,更紧密分布的氧化电位,并且其指示3b 2+和3c 2+的后续反应更快。在中性化合物和它们的单和双氧化产物的计算的电荷分布的变化证实在Mn-Co共ν的移位测量趋势CO频率在主要分配氧化还原位点到diarylbutene片段。除去电子后,损失的电荷密度被可极化的半胱氨酸半胱氨酸标签部分补偿。在室温下,二氯甲烷/0.05 M [NBu 4 ] [B(C 6 F)中,药物3b 2+和3c 2+的半衰期约为10 s。5)4 ]。它们的后续反应通过从二芳基丁烯单元的羟基或CH 2基团损失质子而引发,从而产生具有甲基化醌结构的两种产物。尽管环丙三烯标记的二芳基丁烯的初始氧化部位主要基于配体,而二茂铁标记的二芳基丁烯的起始氧化部位基于金属,但它们的对-OH-或对-OMe官能化衍生物的最终氧化产物非常相似。
更新日期:2020-02-20
中文翻译:

Cymantrene标记的他莫昔芬类似物的氧化:联苯功能化对氧化还原机制的影响。
1,1'-二-的氧化p -anisolyl -2- cymantrenylbutene(图3b)和1,1'-二- p羟基苯基-2- cymantrenylbutene(3C)通过电化学和光谱实验和通过密度泛函理论研究(DFT)计算。两种化合物均进行可逆的单电子氧化,然后进行部分化学可逆的第二次氧化(相对于二茂铁,E 1/2值:3b为0.60和0.74 V ;3c为0.63和0.78 V )。与非苯基官能化母体相比,1,1'-二苯基-2-环丁烯基丁烯(3a),3b,c具有更低,更紧密分布的氧化电位,并且其指示3b 2+和3c 2+的后续反应更快。在中性化合物和它们的单和双氧化产物的计算的电荷分布的变化证实在Mn-Co共ν的移位测量趋势CO频率在主要分配氧化还原位点到diarylbutene片段。除去电子后,损失的电荷密度被可极化的半胱氨酸半胱氨酸标签部分补偿。在室温下,二氯甲烷/0.05 M [NBu 4 ] [B(C 6 F)中,药物3b 2+和3c 2+的半衰期约为10 s。5)4 ]。它们的后续反应通过从二芳基丁烯单元的羟基或CH 2基团损失质子而引发,从而产生具有甲基化醌结构的两种产物。尽管环丙三烯标记的二芳基丁烯的初始氧化部位主要基于配体,而二茂铁标记的二芳基丁烯的起始氧化部位基于金属,但它们的对-OH-或对-OMe官能化衍生物的最终氧化产物非常相似。











































 京公网安备 11010802027423号
京公网安备 11010802027423号