当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation and Characterization of a New Solid Form of Praziquantel, an Essential Anthelmintic Drug. Praziquantel Racemic Monohydrate.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.ejps.2020.105267 Duvernis Salazar-Rojas 1 , Rubén M Maggio 1 , Teodoro S Kaufman 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.ejps.2020.105267 Duvernis Salazar-Rojas 1 , Rubén M Maggio 1 , Teodoro S Kaufman 1
Affiliation
|
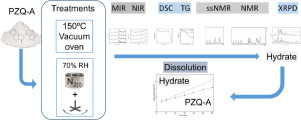
Praziquantel (PZQ) is a highly effective low-cost anthelmintic agent used as the first-choice treatment against schistosomiasis. The low solubility of the active is a major drawback for pharmaceutical formulation. A valid approach of the pharmaceutical industry for the improvement of the pharmacotechnical features of the active principles (such as solubility, processability, stability, among others), is the preparation of new solid forms, such as salts, polymorph, and pseudo-polymorph. Herein we report the preparation and characterization of a new solid form PZQ. The PZQ monohydrate (PZQ-MH) was prepared by a solventless procedure from the commercial racemate and the product was characterized at the solid-state employing optical digital microscopy, thermal methods (melting point, differential scanning calorimetry and thermogravimetric analysis), as weel as and mid-infrared and near infrared spectroscopies. The chemical structure and content of water were full assessed by 1H nuclear magnetic resonance (NMR) in solution. The amount of water in PZQ-was also determined by different approaches, including thermogravimetric analysis and the loss on drying test. Solid-state 13C NMR (ssNMR) and X-ray powder diffraction (XRPD) completed the structural characterization of the new monohydrate. PZQ-MH showed a crystalline behaviour during XRPD experiments and showed relevant differences in spectroscopic, calorimetric, ssNMR and XRPD signals when it was compared with the known crystal (Form A) and amorphous forms of PZQ. The determination of the intrinsic dissolution rate (IDR) of PZQ-MH was carried out as a functional characterization, observing that the new form had slightly higher IDR than Form A.
中文翻译:

吡喹酮(一种必需的驱虫药)新固体形式的制备和表征。吡喹酮消旋一水合物。
吡喹酮(PZQ)是一种高效低成本的驱虫药,被用作抗血吸虫病的首选药物。活性物质的低溶解度是药物制剂的主要缺点。制药工业用于改善活性成分的药理学特征(例如溶解度,可加工性,稳定性等)的有效方法是制备新的固体形式,例如盐,多晶型物和假多晶型物。在这里,我们报告了一种新型固体PZQ的制备和表征。通过无溶剂方法从市售外消旋体制得PZQ一水合物(PZQ-MH),并使用光学数字显微镜,热方法(熔点,差示扫描量热法和热重分析)以固态对产物进行表征。像中红外和近红外光谱一样。通过溶液中的1H核磁共振(NMR)全面评估了水的化学结构和含量。PZQ-中的水量还通过不同的方法确定,包括热重分析和干燥失重测试。固态13C NMR(ssNMR)和X射线粉末衍射(XRPD)完成了新一水合物的结构表征。PZQ-MH在XRPD实验期间显示出结晶行为,并且与已知的PZQ晶体(晶型A)和非晶形形式进行比较时,在光谱,量热,ssNMR和XRPD信号方面显示出相关差异。确定PZQ-MH的固有溶出度(IDR)是作为功能表征进行的,观察到新形式的IDR略高于形式A.
更新日期:2020-02-20
中文翻译:

吡喹酮(一种必需的驱虫药)新固体形式的制备和表征。吡喹酮消旋一水合物。
吡喹酮(PZQ)是一种高效低成本的驱虫药,被用作抗血吸虫病的首选药物。活性物质的低溶解度是药物制剂的主要缺点。制药工业用于改善活性成分的药理学特征(例如溶解度,可加工性,稳定性等)的有效方法是制备新的固体形式,例如盐,多晶型物和假多晶型物。在这里,我们报告了一种新型固体PZQ的制备和表征。通过无溶剂方法从市售外消旋体制得PZQ一水合物(PZQ-MH),并使用光学数字显微镜,热方法(熔点,差示扫描量热法和热重分析)以固态对产物进行表征。像中红外和近红外光谱一样。通过溶液中的1H核磁共振(NMR)全面评估了水的化学结构和含量。PZQ-中的水量还通过不同的方法确定,包括热重分析和干燥失重测试。固态13C NMR(ssNMR)和X射线粉末衍射(XRPD)完成了新一水合物的结构表征。PZQ-MH在XRPD实验期间显示出结晶行为,并且与已知的PZQ晶体(晶型A)和非晶形形式进行比较时,在光谱,量热,ssNMR和XRPD信号方面显示出相关差异。确定PZQ-MH的固有溶出度(IDR)是作为功能表征进行的,观察到新形式的IDR略高于形式A.











































 京公网安备 11010802027423号
京公网安备 11010802027423号