当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CD4-binding obstacles in conformational transitions and allosteric communications of HIV gp120.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.bbamem.2020.183217 Yi Li 1 , Yu-Chen Guo 1 , Xiao-Ling Zhang 1 , Lei Deng 2 , Peng Sang 3 , Li-Quan Yang 3 , Shu-Qun Liu 2
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.bbamem.2020.183217 Yi Li 1 , Yu-Chen Guo 1 , Xiao-Ling Zhang 1 , Lei Deng 2 , Peng Sang 3 , Li-Quan Yang 3 , Shu-Qun Liu 2
Affiliation
|
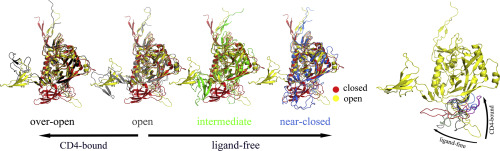
As the only exposed viral protein at the membrane surface of HIV, envelope glycoprotein gp120 is responsible for recognizing host cells and mediating virus-cell membrane fusion. Available structures of gp120 indicate that it exhibits two distinct conformational states, called closed and open states. Although experimental data demonstrates that CD4 binding stabilizes open state of gp120, detailed structural dynamics and kinetics of gp120 during this process remain elusive. Here, two open-state gp120 simulation systems, one without any ligands (ligand-free) and the other complexed with CD4 (CD4-bound), were subjected to microsecond-scale molecular dynamics simulations following the conformational transitions and allosteric pathways of gp120 evaluated by using the Markov state model and a network-based method, respectively. Our results provide an atomic-resolution description of gp120 conformational transitions, suggesting that gp120 is intrinsically dynamic from the open state to closed state, whereas CD4 binding blocks these transitions. Consistent with experimental structures, five metastable conformations with different orientations of the V1/V2 region and V3 loop have been extracted. The binding of CD4 significantly enhances allosteric communications from the CD4-binding site to V3 loop and β20-21 hairpin, resulting in high-affinity interactions with coreceptors and activation of the conformational transitions switcher, respectively. This study will facilitate the structural understanding of the CD4-binding effects on conformational transitions and allosteric pathways of gp120.
中文翻译:

HIV gp120构象转换和变构通讯中的CD4结合障碍。
作为HIV膜表面上唯一暴露的病毒蛋白,包膜糖蛋白gp120负责识别宿主细胞并介导病毒-细胞膜融合。gp120的可用结构表明,它表现出两种不同的构象状态,称为封闭状态和开放状态。尽管实验数据表明CD4结合可稳定gp120的开放状态,但在此过程中gp120的详细结构动力学和动力学仍然难以捉摸。在此,根据评估的gp120的构象转变和变构途径,对两个开放态gp120模拟系统进行了微秒级分子动力学模拟,其中一个没有任何配体(不含配体),另一个与CD4复合(CD4结合)。分别使用马尔可夫状态模型和基于网络的方法。我们的结果提供了gp120构象转变的原子分辨率描述,表明gp120从打开状态到关闭状态本质上是动态的,而CD4结合阻止了这些转变。与实验结构一致,已提取了五个V1 / V2区域和V3环方向不同的亚稳构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。提示gp120从打开状态到关闭状态本质上是动态的,而CD4绑定阻止了这些转换。与实验结构一致,已提取了五个V1 / V2区域和V3环方向不同的亚稳构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。提示gp120从打开状态到关闭状态本质上是动态的,而CD4绑定阻止了这些转换。与实验结构一致,已提取了五个V1 / V2区域和V3环方向不同的亚稳构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。提取了五个具有V1 / V2区域和V3环方向不同的亚稳态构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。提取了五个具有V1 / V2区域和V3环方向不同的亚稳态构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。
更新日期:2020-02-20
中文翻译:

HIV gp120构象转换和变构通讯中的CD4结合障碍。
作为HIV膜表面上唯一暴露的病毒蛋白,包膜糖蛋白gp120负责识别宿主细胞并介导病毒-细胞膜融合。gp120的可用结构表明,它表现出两种不同的构象状态,称为封闭状态和开放状态。尽管实验数据表明CD4结合可稳定gp120的开放状态,但在此过程中gp120的详细结构动力学和动力学仍然难以捉摸。在此,根据评估的gp120的构象转变和变构途径,对两个开放态gp120模拟系统进行了微秒级分子动力学模拟,其中一个没有任何配体(不含配体),另一个与CD4复合(CD4结合)。分别使用马尔可夫状态模型和基于网络的方法。我们的结果提供了gp120构象转变的原子分辨率描述,表明gp120从打开状态到关闭状态本质上是动态的,而CD4结合阻止了这些转变。与实验结构一致,已提取了五个V1 / V2区域和V3环方向不同的亚稳构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。提示gp120从打开状态到关闭状态本质上是动态的,而CD4绑定阻止了这些转换。与实验结构一致,已提取了五个V1 / V2区域和V3环方向不同的亚稳构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。提示gp120从打开状态到关闭状态本质上是动态的,而CD4绑定阻止了这些转换。与实验结构一致,已提取了五个V1 / V2区域和V3环方向不同的亚稳构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。提取了五个具有V1 / V2区域和V3环方向不同的亚稳态构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。提取了五个具有V1 / V2区域和V3环方向不同的亚稳态构象。CD4的结合显着增强了从CD4结合位点到V3环和β20-21发夹的变构通讯,分别导致与共受体的高亲和力相互作用和构象转换开关的激活。这项研究将有助于对gp120的构象转变和变构途径的CD4结合作用的结构理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号