当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immobilization and stabilization of d-hydantoinase from Vigna angularis and its use in the production of N-carbamoyl-d-phenylglycine. Improvement of the reaction yield by allowing chemical racemization of the substrate
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.procbio.2020.02.017 Aline Aparecida Becaro , Adriano Aguiar Mendes , Wellington Sabino Adriano , Laiane Antunes Lopes , Kenia Lourenço Vanzolini , Roberto Fernandez-Lafuente , Paulo Waldir Tardioli , Quezia Bezerra Cass , Raquel de Lima Camargo Giordano
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.procbio.2020.02.017 Aline Aparecida Becaro , Adriano Aguiar Mendes , Wellington Sabino Adriano , Laiane Antunes Lopes , Kenia Lourenço Vanzolini , Roberto Fernandez-Lafuente , Paulo Waldir Tardioli , Quezia Bezerra Cass , Raquel de Lima Camargo Giordano
|
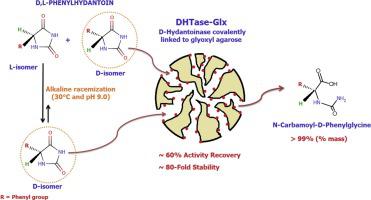
Abstract This work reports the immobilization of a multimeric d -hydantoinase (DHTase) from Vigna angularis (E.C. 3.5.2.2.) on agarose beads activated with glyoxyl groups aiming to improve its stability via multipoint covalent attachment. The final reduction with sodium borohydride resulted in a drop in enzyme activity that could be decreased by adding Zn2+ or Mg2+. The optimal preparation with high activity (58 % recovered activity) and stability (around 86-fold more stable than the free enzyme) was obtained by DHTase immobilization on glyoxyl agarose for 24 h at 25 °C and pH 10.05, and a borohydride reduction step in the presence of 10 mM Zn2+ (DHTase-Glx). The enzyme was almost fully immobilized on glyoxyl agarose (19.8 mg/g of support) when offering 20 mg/g. This immobilized biocatalyst was used to catalyze the hydrolysis of d , l -phenylhydantoin under substrate racemization conditions, which produced 99 % of N-carbamoyl- d -phenylglycine after 9 h reaction.
中文翻译:

来自豇豆的 d-乙内酰脲酶的固定化和稳定化及其在 N-氨基甲酰基-d-苯基甘氨酸生产中的用途。通过允许底物的化学外消旋化来提高反应产率
摘要 这项工作报告了来自 Vigna angularis (EC 3.5.2.2.) 的多聚体 d-乙内酰脲酶 (DHTase) 固定在用乙醛基团激活的琼脂糖珠上,旨在通过多点共价连接提高其稳定性。最后用硼氢化钠还原导致酶活性下降,这可以通过添加 Zn2+ 或 Mg2+ 来降低。通过在 25 °C 和 pH 10.05 下将 DHTase 固定在乙醛琼脂糖上 24 小时,并进行硼氢化物还原步骤,获得了具有高活性(58% 恢复活性)和稳定性(比游离酶稳定约 86 倍)的最佳制剂在 10 mM Zn2+ (DHTase-Glx) 存在下。当提供 20 mg/g 时,该酶几乎完全固定在乙醛琼脂糖(19.8 mg/g 载体)上。这种固定化的生物催化剂用于催化 d 的水解,
更新日期:2020-08-01
中文翻译:

来自豇豆的 d-乙内酰脲酶的固定化和稳定化及其在 N-氨基甲酰基-d-苯基甘氨酸生产中的用途。通过允许底物的化学外消旋化来提高反应产率
摘要 这项工作报告了来自 Vigna angularis (EC 3.5.2.2.) 的多聚体 d-乙内酰脲酶 (DHTase) 固定在用乙醛基团激活的琼脂糖珠上,旨在通过多点共价连接提高其稳定性。最后用硼氢化钠还原导致酶活性下降,这可以通过添加 Zn2+ 或 Mg2+ 来降低。通过在 25 °C 和 pH 10.05 下将 DHTase 固定在乙醛琼脂糖上 24 小时,并进行硼氢化物还原步骤,获得了具有高活性(58% 恢复活性)和稳定性(比游离酶稳定约 86 倍)的最佳制剂在 10 mM Zn2+ (DHTase-Glx) 存在下。当提供 20 mg/g 时,该酶几乎完全固定在乙醛琼脂糖(19.8 mg/g 载体)上。这种固定化的生物催化剂用于催化 d 的水解,











































 京公网安备 11010802027423号
京公网安备 11010802027423号