Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Effects and Functional Implications of Phalloidin and Jasplakinolide Binding to Actin Filaments.
Structure ( IF 4.4 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.str.2020.01.014 Sabrina Pospich 1 , Felipe Merino 1 , Stefan Raunser 1
Structure ( IF 4.4 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.str.2020.01.014 Sabrina Pospich 1 , Felipe Merino 1 , Stefan Raunser 1
Affiliation
|
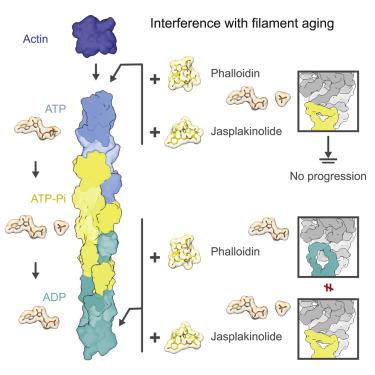
Actin undergoes structural transitions during polymerization, ATP hydrolysis, and subsequent release of inorganic phosphate. Several actin-binding proteins sense specific states during this transition and can thus target different regions of the actin filament. Here, we show in atomic detail that phalloidin, a mushroom toxin that is routinely used to stabilize and label actin filaments, suspends the structural changes in actin, likely influencing its interaction with actin-binding proteins. Furthermore, high-resolution cryoelectron microscopy structures reveal structural rearrangements in F-actin upon inorganic phosphate release in phalloidin-stabilized filaments. We find that the effect of the sponge toxin jasplakinolide differs from the one of phalloidin, despite their overlapping binding site and similar interactions with the actin filament. Analysis of structural conformations of F-actin suggests that stabilizing agents trap states within the natural conformational space of actin.
中文翻译:

鬼笔环肽和Jasplakinolide结合到肌动蛋白丝的结构效应和功能含义。
肌动蛋白在聚合,ATP水解以及随后的无机磷酸盐释放过程中经历结构转变。几种肌动蛋白结合蛋白在此过渡过程中感觉到特定状态,因此可以靶向肌动蛋白丝的不同区域。在这里,我们在原子细节上显示,鬼笔环肽是一种蘑菇毒素,通常用于稳定和标记肌动蛋白丝,会暂停肌动蛋白的结构变化,可能会影响其与肌动蛋白结合蛋白的相互作用。此外,高分辨率的冷冻电子显微镜结构揭示了在鬼笔环肽稳定的细丝中释放无机磷酸盐后F-肌动蛋白的结构重排。我们发现海绵毒素jasplakinolide的作用不同于鬼笔环肽,尽管它们的结合位点重叠且与肌动蛋白丝的相互作用相似。
更新日期:2020-02-20
中文翻译:

鬼笔环肽和Jasplakinolide结合到肌动蛋白丝的结构效应和功能含义。
肌动蛋白在聚合,ATP水解以及随后的无机磷酸盐释放过程中经历结构转变。几种肌动蛋白结合蛋白在此过渡过程中感觉到特定状态,因此可以靶向肌动蛋白丝的不同区域。在这里,我们在原子细节上显示,鬼笔环肽是一种蘑菇毒素,通常用于稳定和标记肌动蛋白丝,会暂停肌动蛋白的结构变化,可能会影响其与肌动蛋白结合蛋白的相互作用。此外,高分辨率的冷冻电子显微镜结构揭示了在鬼笔环肽稳定的细丝中释放无机磷酸盐后F-肌动蛋白的结构重排。我们发现海绵毒素jasplakinolide的作用不同于鬼笔环肽,尽管它们的结合位点重叠且与肌动蛋白丝的相互作用相似。











































 京公网安备 11010802027423号
京公网安备 11010802027423号