Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of Microtubule-Trapped Human Kinesin-5 and Its Mechanism of Inhibition Revealed Using Cryoelectron Microscopy.
Structure ( IF 4.4 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.str.2020.01.013 Alejandro Peña 1 , Aaron Sweeney 1 , Alexander D Cook 1 , Julia Locke 1 , Maya Topf 1 , Carolyn A Moores 1
Structure ( IF 4.4 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.str.2020.01.013 Alejandro Peña 1 , Aaron Sweeney 1 , Alexander D Cook 1 , Julia Locke 1 , Maya Topf 1 , Carolyn A Moores 1
Affiliation
|
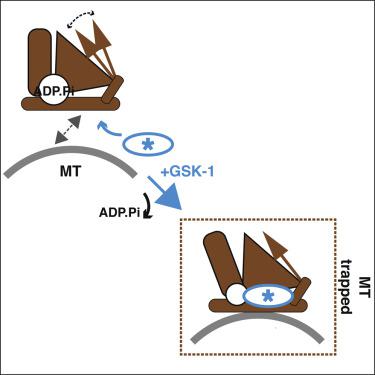
Kinesin-5 motors are vital mitotic spindle components, and disruption of their function perturbs cell division. We investigated the molecular mechanism of the human kinesin-5 inhibitor GSK-1, which allosterically promotes tight microtubule binding. GSK-1 inhibits monomeric human kinesin-5 ATPase and microtubule gliding activities, and promotes the motor's microtubule stabilization activity. Using cryoelectron microscopy, we determined the 3D structure of the microtubule-bound motor-GSK-1 at 3.8 Å overall resolution. The structure reveals that GSK-1 stabilizes the microtubule binding surface of the motor in an ATP-like conformation, while destabilizing regions of the motor around the empty nucleotide binding pocket. Density corresponding to GSK-1 is located between helix-α4 and helix-α6 in the motor domain at its interface with the microtubule. Using a combination of difference mapping and protein-ligand docking, we characterized the kinesin-5-GSK-1 interaction and further validated this binding site using mutagenesis. This work opens up new avenues of investigation of kinesin inhibition and spindle perturbation.
中文翻译:

使用冷冻电子显微镜揭示微管捕获的人驱动蛋白-5 的结构及其抑制机制。
Kinesin-5 马达是重要的有丝分裂纺锤体组件,其功能的破坏会扰乱细胞分裂。我们研究了人驱动蛋白 5 抑制剂 GSK-1 的分子机制,该抑制剂以变构方式促进紧密的微管结合。GSK-1 抑制单体人驱动蛋白 5 ATP 酶和微管滑动活性,并促进马达的微管稳定活性。使用冷冻电子显微镜,我们以 3.8 Å 的整体分辨率确定了微管结合的马达 GSK-1 的 3D 结构。该结构揭示了 GSK-1 使马达的微管结合表面稳定在 ATP 样构象中,同时使空核苷酸结合袋周围的马达区域不稳定。与 GSK-1 相对应的密度位于运动域中螺旋-α4 和螺旋-α6 之间,位于其与微管的界面处。通过结合差异作图和蛋白质-配体对接,我们表征了驱动蛋白-5-GSK-1 相互作用,并利用诱变进一步验证了该结合位点。这项工作开辟了研究驱动蛋白抑制和纺锤体扰动的新途径。
更新日期:2020-02-20
中文翻译:

使用冷冻电子显微镜揭示微管捕获的人驱动蛋白-5 的结构及其抑制机制。
Kinesin-5 马达是重要的有丝分裂纺锤体组件,其功能的破坏会扰乱细胞分裂。我们研究了人驱动蛋白 5 抑制剂 GSK-1 的分子机制,该抑制剂以变构方式促进紧密的微管结合。GSK-1 抑制单体人驱动蛋白 5 ATP 酶和微管滑动活性,并促进马达的微管稳定活性。使用冷冻电子显微镜,我们以 3.8 Å 的整体分辨率确定了微管结合的马达 GSK-1 的 3D 结构。该结构揭示了 GSK-1 使马达的微管结合表面稳定在 ATP 样构象中,同时使空核苷酸结合袋周围的马达区域不稳定。与 GSK-1 相对应的密度位于运动域中螺旋-α4 和螺旋-α6 之间,位于其与微管的界面处。通过结合差异作图和蛋白质-配体对接,我们表征了驱动蛋白-5-GSK-1 相互作用,并利用诱变进一步验证了该结合位点。这项工作开辟了研究驱动蛋白抑制和纺锤体扰动的新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号