当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissociation of Adaptive Thermogenesis from Glucose Homeostasis in Microbiome-Deficient Mice.
Cell Metabolism ( IF 27.7 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.cmet.2020.01.012 Tibor I Krisko 1 , Hayley T Nicholls 1 , Curtis J Bare 1 , Corey D Holman 1 , Gregory G Putzel 2 , Robert S Jansen 3 , Natalie Sun 1 , Kyu Y Rhee 3 , Alexander S Banks 4 , David E Cohen 1
Cell Metabolism ( IF 27.7 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.cmet.2020.01.012 Tibor I Krisko 1 , Hayley T Nicholls 1 , Curtis J Bare 1 , Corey D Holman 1 , Gregory G Putzel 2 , Robert S Jansen 3 , Natalie Sun 1 , Kyu Y Rhee 3 , Alexander S Banks 4 , David E Cohen 1
Affiliation
|
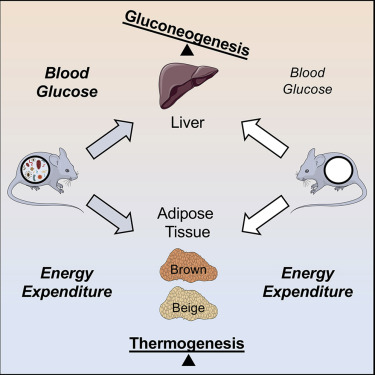
Recent studies suggest that a key mechanism whereby the gut microbiome influences energy balance and glucose homeostasis is through the recruitment of brown and beige adipocytes, primary mediators of the adaptive thermogenic response. To test this, we assessed energy expenditure and glucose metabolism in two complementary mouse models of gut microbial deficiency, which were exposed to a broad range of thermal and dietary stresses. Neither ablation of the gut microbiome, nor the substantial microbial perturbations induced by cold ambient temperatures, influenced energy expenditure during cold exposure or high-fat feeding. Nevertheless, we demonstrated a critical role for gut microbial metabolism in maintaining euglycemia through the production of amino acid metabolites that optimized hepatic TCA (tricarboxylic acid) cycle fluxes in support of gluconeogenesis. These results distinguish the dispensability of the gut microbiome for the regulation of energy expenditure from its critical contribution to the maintenance of glucose homeostasis.
中文翻译:

在微生物组缺陷型小鼠中从葡萄糖稳态中解离自适应生热作用。
最近的研究表明,肠道微生物组影响能量平衡和葡萄糖稳态的关键机制是通过募集棕色和米色的脂肪细胞,它们是适应性产热反应的主要介质。为了测试这一点,我们在两种补充的肠道微生物缺陷小鼠模型中评估了能量消耗和葡萄糖代谢,这两种模型暴露于广泛的热应激和饮食压力下。肠道微生物组的消融或寒冷环境温度引起的大量微生物摄动都不会影响冷暴露或高脂喂养期间的能量消耗。不过,我们证明了通过优化氨基酸TCA(三羧酸)循环通量以支持糖异生的氨基酸代谢产物,肠道微生物代谢在维持正常血糖方面具有关键作用。这些结果区别了肠道微生物组在调节能量消耗方面的可分配性及其对维持葡萄糖稳态的关键作用。
更新日期:2020-02-20
中文翻译:

在微生物组缺陷型小鼠中从葡萄糖稳态中解离自适应生热作用。
最近的研究表明,肠道微生物组影响能量平衡和葡萄糖稳态的关键机制是通过募集棕色和米色的脂肪细胞,它们是适应性产热反应的主要介质。为了测试这一点,我们在两种补充的肠道微生物缺陷小鼠模型中评估了能量消耗和葡萄糖代谢,这两种模型暴露于广泛的热应激和饮食压力下。肠道微生物组的消融或寒冷环境温度引起的大量微生物摄动都不会影响冷暴露或高脂喂养期间的能量消耗。不过,我们证明了通过优化氨基酸TCA(三羧酸)循环通量以支持糖异生的氨基酸代谢产物,肠道微生物代谢在维持正常血糖方面具有关键作用。这些结果区别了肠道微生物组在调节能量消耗方面的可分配性及其对维持葡萄糖稳态的关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号