当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetrically Segregated Mitochondria Provide Cellular Memory of Hematopoietic Stem Cell Replicative History and Drive HSC Attrition.
Cell Stem Cell ( IF 19.8 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.stem.2020.01.016 Ashwini Hinge 1 , Jingyi He 2 , James Bartram 1 , Jose Javier 1 , Juying Xu 1 , Ellen Fjellman 1 , Hiromi Sesaki 3 , Tingyu Li 4 , Jie Yu 5 , Mark Wunderlich 1 , James Mulloy 1 , Matthew Kofron 6 , Nathan Salomonis 7 , H Leighton Grimes 8 , Marie-Dominique Filippi 1
Cell Stem Cell ( IF 19.8 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.stem.2020.01.016 Ashwini Hinge 1 , Jingyi He 2 , James Bartram 1 , Jose Javier 1 , Juying Xu 1 , Ellen Fjellman 1 , Hiromi Sesaki 3 , Tingyu Li 4 , Jie Yu 5 , Mark Wunderlich 1 , James Mulloy 1 , Matthew Kofron 6 , Nathan Salomonis 7 , H Leighton Grimes 8 , Marie-Dominique Filippi 1
Affiliation
|
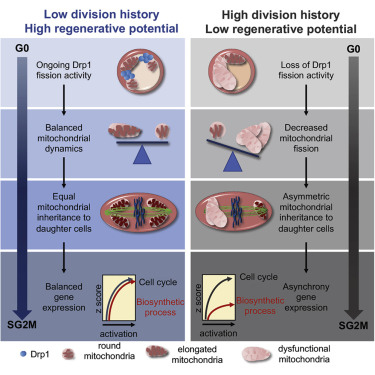
The metabolic requirements of hematopoietic stem cells (HSCs) change with their cell cycle activity. However, the underlying role of mitochondria remains ill-defined. Here we found that, after mitochondrial activation with replication, HSCs irreversibly remodel the mitochondrial network and that this network is not repaired after HSC re-entry into quiescence, contrary to hematopoietic progenitors. HSCs keep and accumulate dysfunctional mitochondria through asymmetric segregation during active division. Mechanistically, mitochondria aggregate and depolarize after stress because of loss of activity of the mitochondrial fission regulator Drp1 onto mitochondria. Genetic and pharmacological studies indicate that inactivation of Drp1 causes loss of HSC regenerative potential while maintaining HSC quiescence. Molecularly, HSCs carrying dysfunctional mitochondria can re-enter quiescence but fail to synchronize the transcriptional control of core cell cycle and metabolic components in subsequent division. Thus, loss of fidelity of mitochondrial morphology and segregation is one type of HSC divisional memory and drives HSC attrition.
中文翻译:

不对称分离的线粒体提供了造血干细胞复制历史的细胞记忆并驱动了HSC磨损。
造血干细胞(HSC)的代谢要求随其细胞周期活性而变化。但是,线粒体的潜在作用仍然不清楚。在这里,我们发现,线粒体激活并复制后,HSC不可逆地重塑了线粒体网络,并且与造血祖细胞相反,HSC重新进入静止状态后该网络未得到修复。HSC通过主动分裂过程中的不对称分离来保持和积累功能异常的线粒体。从机理上讲,线粒体在应力后聚集并去极化,这是因为线粒体裂变调节剂Drp1在线粒体上的活性丧失。遗传和药理研究表明,Drp1的失活会导致HSC再生潜能的丧失,同时保持HSC静止。在分子上,携带功能异常的线粒体的HSC可以重新进入静止状态,但在随后的分裂中无法同步核心细胞周期和代谢成分的转录控制。因此,线粒体形态保真性和分离的丧失是HSC分区记忆的一种类型,并驱动HSC磨损。
更新日期:2020-02-20
中文翻译:

不对称分离的线粒体提供了造血干细胞复制历史的细胞记忆并驱动了HSC磨损。
造血干细胞(HSC)的代谢要求随其细胞周期活性而变化。但是,线粒体的潜在作用仍然不清楚。在这里,我们发现,线粒体激活并复制后,HSC不可逆地重塑了线粒体网络,并且与造血祖细胞相反,HSC重新进入静止状态后该网络未得到修复。HSC通过主动分裂过程中的不对称分离来保持和积累功能异常的线粒体。从机理上讲,线粒体在应力后聚集并去极化,这是因为线粒体裂变调节剂Drp1在线粒体上的活性丧失。遗传和药理研究表明,Drp1的失活会导致HSC再生潜能的丧失,同时保持HSC静止。在分子上,携带功能异常的线粒体的HSC可以重新进入静止状态,但在随后的分裂中无法同步核心细胞周期和代谢成分的转录控制。因此,线粒体形态保真性和分离的丧失是HSC分区记忆的一种类型,并驱动HSC磨损。











































 京公网安备 11010802027423号
京公网安备 11010802027423号