当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and biological evaluation of 4,5-dihydro-[1,2,4]triazolo[4,3-f]pteridine derivatives as novel dual-PLK1/BRD4 inhibitors.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.ejmech.2020.112152 Ning-Yu Wang 1 , Ying Xu 2 , Kun-Jie Xiao 2 , Wei-Qiong Zuo 2 , Yong-Xia Zhu 2 , Rong Hu 1 , Wan-Li Wang 1 , Yao-Jie Shi 2 , Luo-Ting Yu 2 , Zhi-Hao Liu 2
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.ejmech.2020.112152 Ning-Yu Wang 1 , Ying Xu 2 , Kun-Jie Xiao 2 , Wei-Qiong Zuo 2 , Yong-Xia Zhu 2 , Rong Hu 1 , Wan-Li Wang 1 , Yao-Jie Shi 2 , Luo-Ting Yu 2 , Zhi-Hao Liu 2
Affiliation
|
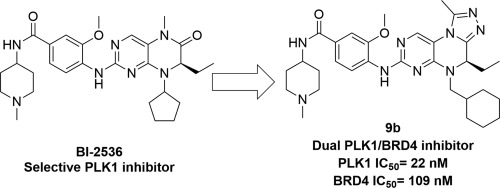
Protein kinase inhibitors and epigenetic regulatory molecules are two main kinds of anticancer drugs developed in recent years. Both kinds of drugs harbor their own advantages and disadvantages in the treatment of cancer, and the development of small molecules which could target at kinases and epigenetic targets simultaneously can avoid the defects of drugs which only targets at kinases or epigenetic proteins. In this study, a series of 4,5-dihydro-[1,2,4]triazolo [4,3-f]pteridine derivatives were designed and synthesized based on the structure of PLK1 inhibitor BI-2536. Subsequent targets affinity screen and antiproliferative activity test led to the discovery of the most potent dual PLK1/BRD4 inhibitor 9b with good potency for both PLK1 (IC50 = 22 nM) and BRD4 (IC50 = 109 nM) as well as favorable antiproliferative activity against a panel of cancer cell lines. 9b could induce cell cycle arrest and apoptosis in acute myeloid leukemia cell line MV 4-11 in a concentration dependent manner. It could also downregulate the transcription of several proliferation-related oncogenes, including c-MYC, MYCN and BCL-2. Finally, in a MV4-11 mouse xenograft model, 9b exhibited favorable in vivo antitumor activity with 66% tumor growth inhibition (TGI) at a dose of 60 mg/kg while without obvious toxicity. This study thus provided us a start point for the development of new dual PLK1/BRD4 inhibitors as anticancer agents.
中文翻译:

作为新型双 PLK1/BRD4 抑制剂的 4,5-二氢-[1,2,4]三唑并[4,3-f]蝶啶衍生物的设计、合成和生物学评价。
蛋白激酶抑制剂和表观遗传调控分子是近年来开发的两大类抗癌药物。两种药物在治疗癌症方面各有优缺点,开发同时靶向激酶和表观遗传靶点的小分子可以避免仅靶向激酶或表观遗传蛋白药物的缺陷。本研究基于PLK1抑制剂BI-2536的结构,设计合成了一系列4,5-二氢-[1,2,4]三唑并[4,3-f]蝶啶衍生物。随后的靶标亲和力筛选和抗增殖活性测试发现了最有效的双重 PLK1/BRD4 抑制剂 9b,对 PLK1 (IC50 = 22 nM) 和 BRD4 (IC50 = 109 nM) 均具有良好的效力,并且对癌细胞系组。 9b能够以浓度依赖性方式诱导急性髓性白血病细胞系MV 4-11的细胞周期停滞和细胞凋亡。它还可以下调多种增殖相关癌基因的转录,包括 c-MYC、MYCN 和 BCL-2。最后,在MV4-11小鼠异种移植模型中,9b表现出良好的体内抗肿瘤活性,在60 mg/kg的剂量下,肿瘤生长抑制(TGI)达到66%,同时没有明显的毒性。因此,这项研究为我们开发新型 PLK1/BRD4 双重抑制剂作为抗癌药物提供了一个起点。
更新日期:2020-02-20
中文翻译:

作为新型双 PLK1/BRD4 抑制剂的 4,5-二氢-[1,2,4]三唑并[4,3-f]蝶啶衍生物的设计、合成和生物学评价。
蛋白激酶抑制剂和表观遗传调控分子是近年来开发的两大类抗癌药物。两种药物在治疗癌症方面各有优缺点,开发同时靶向激酶和表观遗传靶点的小分子可以避免仅靶向激酶或表观遗传蛋白药物的缺陷。本研究基于PLK1抑制剂BI-2536的结构,设计合成了一系列4,5-二氢-[1,2,4]三唑并[4,3-f]蝶啶衍生物。随后的靶标亲和力筛选和抗增殖活性测试发现了最有效的双重 PLK1/BRD4 抑制剂 9b,对 PLK1 (IC50 = 22 nM) 和 BRD4 (IC50 = 109 nM) 均具有良好的效力,并且对癌细胞系组。 9b能够以浓度依赖性方式诱导急性髓性白血病细胞系MV 4-11的细胞周期停滞和细胞凋亡。它还可以下调多种增殖相关癌基因的转录,包括 c-MYC、MYCN 和 BCL-2。最后,在MV4-11小鼠异种移植模型中,9b表现出良好的体内抗肿瘤活性,在60 mg/kg的剂量下,肿瘤生长抑制(TGI)达到66%,同时没有明显的毒性。因此,这项研究为我们开发新型 PLK1/BRD4 双重抑制剂作为抗癌药物提供了一个起点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号