当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and pharmacological evaluation of 4- or 6-phenyl-pyrimidine derivatives as novel and selective Janus kinase 3 inhibitors.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-16 , DOI: 10.1016/j.ejmech.2020.112148 Lei Shu 1 , Chengjuan Chen 2 , Xueting Huan 1 , Hao Huang 1 , Manman Wang 1 , Jianqiu Zhang 1 , Yile Yan 1 , Jianming Liu 1 , Tiantai Zhang 2 , Dayong Zhang 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-16 , DOI: 10.1016/j.ejmech.2020.112148 Lei Shu 1 , Chengjuan Chen 2 , Xueting Huan 1 , Hao Huang 1 , Manman Wang 1 , Jianqiu Zhang 1 , Yile Yan 1 , Jianming Liu 1 , Tiantai Zhang 2 , Dayong Zhang 1
Affiliation
|
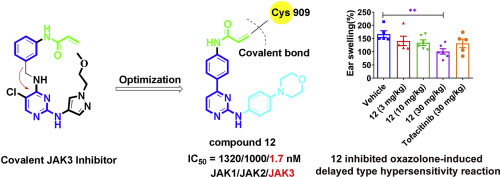
As non-receptor tyrosine kinases, Janus kinases (JAKs) have become an attractive target for the treatment of autoimmune diseases and cancers. JAKs play a pivotal role in innate immunity, inflammation, and hematopoiesis by mediating the signaling of numerous cytokines, growth factors, and interferons (IFNs). Selective inhibitors of a variety of JAK members are expected to inhibit pro-inflammatory cytokine-mediated inflammation and immune responses, while preventing targeting other subtypes of JAKs. In this work, poorly selective compounds based on 4- or 6-phenyl-pyrimidine derivatives have been improved to highly potent and selective compounds by designing a covalent binding tether, which attaches to the unique cysteine (Cys909) residue in JAK3. Compound 12 exhibited potent JAK3 inhibitory activity (IC50 = 1.7 nM) with an excellent selectivity profile when compared to the other JAK isoforms (>588-fold). In a cellular assay, compound 12 strongly inhibited JAK3-dependent signaling and T cell proliferation. Moreover, in vivo data revealed that compound 12 significantly suppressed oxazolone (OXZ)-induced delayed hypersensitivity responses in Balb/c mice. Compound 12 also displayed decent pharmacokinetic properties and was suitable for in vivo use. Taken together, these results indicated that compound 12 may be a promising tool compound as a selective JAK3 inhibitor for treating autoimmune diseases.
中文翻译:

设计,合成和药理学评估的4-或6-苯基嘧啶衍生物作为新型和选择性Janus激酶3抑制剂。
作为非受体酪氨酸激酶,Janus激酶(JAKs)已成为治疗自身免疫性疾病和癌症的有吸引力的靶标。JAK通过介导多种细胞因子,生长因子和干扰素(IFN)的信号传导,在先天免疫,炎症和造血过程中发挥关键作用。各种JAK成员的选择性抑制剂有望抑制促炎性细胞因子介导的炎症和免疫反应,同时防止靶向其他亚型的JAK。在这项工作中,通过设计与JAK3中独特的半胱氨酸(Cys909)残基连接的共价结合链,将基于4-或6-苯基-嘧啶衍生物的选择性差的化合物改进为高效的选择性化合物。化合物12表现出有效的JAK3抑制活性(IC50 = 1。与其他JAK同工型(> 588倍)相比,具有极好的选择性。在细胞分析中,化合物12强烈抑制JAK3依赖性信号传导和T细胞增殖。此外,体内数据显示,化合物12在Balb / c小鼠中显着抑制了恶唑酮(OXZ)诱导的迟发型超敏反应。化合物12还显示出体面的药代动力学性质,并且适合体内使用。综上所述,这些结果表明化合物12作为用于治疗自身免疫疾病的选择性JAK3抑制剂可能是有前途的工具化合物。体内数据显示,化合物12在Balb / c小鼠中显着抑制了恶唑酮(OXZ)诱导的迟发型超敏反应。化合物12还显示出体面的药代动力学性质,并且适合体内使用。综上所述,这些结果表明化合物12作为用于治疗自身免疫疾病的选择性JAK3抑制剂可能是有前途的工具化合物。体内数据显示,化合物12可显着抑制恶唑酮(OXZ)诱导的Balb / c小鼠迟发型超敏反应。化合物12还显示出体面的药代动力学性质,并且适合体内使用。综上所述,这些结果表明化合物12作为用于治疗自身免疫疾病的选择性JAK3抑制剂可能是有前途的工具化合物。
更新日期:2020-02-20
中文翻译:

设计,合成和药理学评估的4-或6-苯基嘧啶衍生物作为新型和选择性Janus激酶3抑制剂。
作为非受体酪氨酸激酶,Janus激酶(JAKs)已成为治疗自身免疫性疾病和癌症的有吸引力的靶标。JAK通过介导多种细胞因子,生长因子和干扰素(IFN)的信号传导,在先天免疫,炎症和造血过程中发挥关键作用。各种JAK成员的选择性抑制剂有望抑制促炎性细胞因子介导的炎症和免疫反应,同时防止靶向其他亚型的JAK。在这项工作中,通过设计与JAK3中独特的半胱氨酸(Cys909)残基连接的共价结合链,将基于4-或6-苯基-嘧啶衍生物的选择性差的化合物改进为高效的选择性化合物。化合物12表现出有效的JAK3抑制活性(IC50 = 1。与其他JAK同工型(> 588倍)相比,具有极好的选择性。在细胞分析中,化合物12强烈抑制JAK3依赖性信号传导和T细胞增殖。此外,体内数据显示,化合物12在Balb / c小鼠中显着抑制了恶唑酮(OXZ)诱导的迟发型超敏反应。化合物12还显示出体面的药代动力学性质,并且适合体内使用。综上所述,这些结果表明化合物12作为用于治疗自身免疫疾病的选择性JAK3抑制剂可能是有前途的工具化合物。体内数据显示,化合物12在Balb / c小鼠中显着抑制了恶唑酮(OXZ)诱导的迟发型超敏反应。化合物12还显示出体面的药代动力学性质,并且适合体内使用。综上所述,这些结果表明化合物12作为用于治疗自身免疫疾病的选择性JAK3抑制剂可能是有前途的工具化合物。体内数据显示,化合物12可显着抑制恶唑酮(OXZ)诱导的Balb / c小鼠迟发型超敏反应。化合物12还显示出体面的药代动力学性质,并且适合体内使用。综上所述,这些结果表明化合物12作为用于治疗自身免疫疾病的选择性JAK3抑制剂可能是有前途的工具化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号