Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.apcata.2020.117471 Milan Hronec , Katarína Fulajtárová , Blažej Horváth , Tibor Liptaj , Edmund Dobročka
|
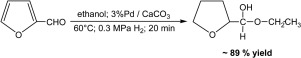
An entirely new and highly selective method for preparation of novel tetrahydrofurfuryl hemiacetals is described. The process is based on the catalytic hydrogenation of furfural in an alcohol under mild reaction conditions and at very short reaction times. As a highly active and selective catalyst palladium supported on calcium carbonate is used. Basic sites of the catalyst support enhance the formation of furfuryl hemiacetal as the intermediate which is instantaneously hydrogenated into stable tetrahydrofurfuryl hemiacetal. About 85 - 90% yields of tetrahydrofurfuryl hemialkylacetals can be achieved within 20 min by reaction of furfural in alcoholic solutions at 60 °C and 0.3 MPa of hydrogen. The mechanism of reductive acetalization of furfural into tetrahydrofurfuryl hemialkylacetals is proposed.
中文翻译:

糠醛向新型四氢糠基半缩醛的轻松转化
描述了一种用于制备新型四氢糠基半缩醛的全新且高度选择性的方法。该方法基于在温和的反应条件下和非常短的反应时间下在醇中糠醛的催化加氢。作为负载在碳酸钙上的钯的高活性和选择性催化剂。催化剂载体的碱性位提高了糠基半缩醛作为中间体的形成,该中间体被立即氢化成稳定的四氢糠基半缩醛。通过使糠醛在60°C和0.3 MPa的乙醇溶液中反应,可在20分钟内获得约85-90%的四氢糠基半烷基缩醛。提出了糠醛还原缩醛化为四氢糠基半烷基缩醛的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号