Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-02-13 , DOI: 10.1016/j.apcata.2020.117467 Giovanni E. Rossi , John M. Winfield , Christopher J. Mitchell , Willem van der Borden , Klaas van der Velde , Robert H. Carr , David Lennon
|
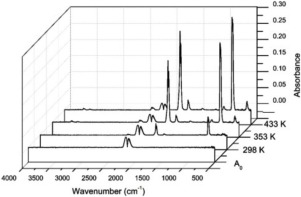
An apparatus is described to investigate the synthesis of phosgene from the reaction of carbon monoxide and dichlorine over an activated carbon catalyst. Infrared spectroscopy and UV-visible absorption spectroscopy are used to identify and quantify reagents and products. The reaction is operated with an excess of CO in order to enable complete chlorine conversion at elevated temperatures. The reaction profile is examined over the temperature range of 300-445 K, with a phosgene selectivity of 100% observed at all temperatures. An isosbestic point in the UV-visible spectrum is observed at 272 nm, indicating that the dichlorine and the phosgene are in equilibrium. Examination of the phosgene formation rate as a function of space time and catalyst size fraction at 323 K establishes that, under the described conditions, the reaction is operating under chemical control in the absence of mass transfer restrictions.
中文翻译:

在活性炭催化剂上通过一氧化碳和二氯反应形成光气:反应测试装置
描述了一种用于研究在活性炭催化剂上由一氧化碳和二氯反应合成光气的装置。红外光谱和紫外可见吸收光谱用于鉴定和定量试剂和产物。该反应用过量的CO进行,以便能够在升高的温度下完全氯转化。在300-445 K的温度范围内检查反应曲线,在所有温度下观察到的光气选择性为100%。在272 nm处观察到UV-可见光谱中的等吸收点,表明二氯和光气处于平衡状态。根据光气形成速率与时空和催化剂尺寸分数在323 K下的关系,可以确定在上述条件下,











































 京公网安备 11010802027423号
京公网安备 11010802027423号