当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioactivation of lumiracoxib in human liver microsomes: Formation of GSH- and amino adducts through acyl glucuronide.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-02-19 , DOI: 10.1002/dta.2777 Weijie Jiao 1 , Xu Zhao 1 , Guiyue Wu 1 , Xiangyun Zhang 1 , Hong Wu 2 , Yinglin Cui 3
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-02-19 , DOI: 10.1002/dta.2777 Weijie Jiao 1 , Xu Zhao 1 , Guiyue Wu 1 , Xiangyun Zhang 1 , Hong Wu 2 , Yinglin Cui 3
Affiliation
|
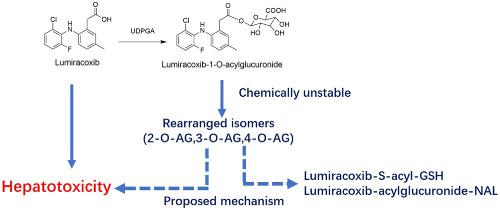
Lumiracoxib is a selective cyclooxygenase‐2 inhibitor, which has been reported to cause rare but severe liver injury. Considering that lumiracoxib has a carboxylic group in the molecule, glucuronidation to form acylglucuronide would be one of the possible mechanisms of lumiracoxib‐induced liver injury. The aim of this study was to identify the metabolites of lumiracoxib that were formed via acyl‐glucuronidation in human liver microsomes using glutathione (GSH) and N‐acetyl‐lysine (NAL) as trapping agents by liquid chromatography combined with high resolution mass spectrometry. The structures of the detected metabolites were identified by their accurate masses, fragment ions, and retention times. Under the current conditions, eight lumiracoxib associated metabolites were identified. With the presence of UDPGA, lumiracoxib was biotransformed into lumiracoxib‐1‐O‐acylglucuronide (M1) and 4′‐hydroxyl‐lumiracoxib‐1‐O‐acylglucuronide (M2), both of which were reactive and prone to react with GSH to form drug‐S‐acyl‐GSH adducts (M3 and M4) through transacylation. In addition to reaction with GSH, the formed 1‐O‐acylglucuronides were chemically unstable (T1/2 = 1.5 h in phosphate buffer) and rearranged to 2‐, 3‐, and/or 4‐isomers, which further underwent ring‐opening to form aldehyde derivatives and then reacted with NAL to yield Schiff base derivatives (M5–M8). The present study provides a clear bioactivation profile of lumiracoxib through acyl glucuronidation, which would be one of the mechanisms attributed to liver injury caused by lumiracoxib.
中文翻译:

人肝微粒体中鲁美昔布的生物活化:通过酰基葡糖醛酸苷形成GSH-和氨基加合物。
Lumiracoxib是一种选择性的环氧合酶2抑制剂,据报道可引起罕见但严重的肝损伤。考虑到lumiracoxib在分子中具有一个羧基,葡萄糖醛酸化形成酰基葡糖醛酸可能是lumiracoxib引起肝损伤的可能机制之一。这项研究的目的是鉴定使用谷胱甘肽(GSH)和N在人肝微粒体中通过酰基葡萄糖醛酸化作用形成的卢美昔布的代谢产物液相色谱与高分辨率质谱联用作为捕获剂的乙酰乙酰赖氨酸(NAL)。检测到的代谢物的结构通过其准确的质量,碎片离子和保留时间来鉴定。在当前条件下,鉴定了八种鲁美昔布相关代谢物。在存在UDPGA的情况下,lumiracoxib被生物转化为lumiracoxib-1-O-酰基葡糖醛酸(M1)和4'-羟基-lumiracoxib-1-O-酰基葡糖醛酸(M2),它们都具有反应性并且易于与GSH反应形成药物S-酰基GSH加合物(M3和M4)通过酰基转移作用。除了与GSH反应外,所形成的1-O-酰基葡糖醛酸苷在化学上也不稳定(T 1/2=在磷酸盐缓冲液中停留1.5小时),然后重排为2、3和/或4个异构体,然后进一步开环形成醛衍生物,然后与NAL反应生成席夫碱衍生物(M5-M8)。本研究通过酰基葡糖醛酸糖化提供了鲁美昔布的明确的生物活化特征,这将是鲁美昔布引起的肝损伤的机制之一。
更新日期:2020-02-19
中文翻译:

人肝微粒体中鲁美昔布的生物活化:通过酰基葡糖醛酸苷形成GSH-和氨基加合物。
Lumiracoxib是一种选择性的环氧合酶2抑制剂,据报道可引起罕见但严重的肝损伤。考虑到lumiracoxib在分子中具有一个羧基,葡萄糖醛酸化形成酰基葡糖醛酸可能是lumiracoxib引起肝损伤的可能机制之一。这项研究的目的是鉴定使用谷胱甘肽(GSH)和N在人肝微粒体中通过酰基葡萄糖醛酸化作用形成的卢美昔布的代谢产物液相色谱与高分辨率质谱联用作为捕获剂的乙酰乙酰赖氨酸(NAL)。检测到的代谢物的结构通过其准确的质量,碎片离子和保留时间来鉴定。在当前条件下,鉴定了八种鲁美昔布相关代谢物。在存在UDPGA的情况下,lumiracoxib被生物转化为lumiracoxib-1-O-酰基葡糖醛酸(M1)和4'-羟基-lumiracoxib-1-O-酰基葡糖醛酸(M2),它们都具有反应性并且易于与GSH反应形成药物S-酰基GSH加合物(M3和M4)通过酰基转移作用。除了与GSH反应外,所形成的1-O-酰基葡糖醛酸苷在化学上也不稳定(T 1/2=在磷酸盐缓冲液中停留1.5小时),然后重排为2、3和/或4个异构体,然后进一步开环形成醛衍生物,然后与NAL反应生成席夫碱衍生物(M5-M8)。本研究通过酰基葡糖醛酸糖化提供了鲁美昔布的明确的生物活化特征,这将是鲁美昔布引起的肝损伤的机制之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号