当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Observation of the Unbiased Conformers of Putative DNA-Scaffold Ribosugars.
ACS Central Science ( IF 12.7 ) Pub Date : 2020-02-18 , DOI: 10.1021/acscentsci.9b01277 Camilla Calabrese 1, 2 , Iciar Uriarte 1, 2 , Aran Insausti 1, 2 , Montserrat Vallejo-López 1 , Francisco J Basterretxea 1 , Stephen A Cochrane 3 , Benjamin G Davis 3, 4 , Francisco Corzana 5 , Emilio J Cocinero 1, 2
ACS Central Science ( IF 12.7 ) Pub Date : 2020-02-18 , DOI: 10.1021/acscentsci.9b01277 Camilla Calabrese 1, 2 , Iciar Uriarte 1, 2 , Aran Insausti 1, 2 , Montserrat Vallejo-López 1 , Francisco J Basterretxea 1 , Stephen A Cochrane 3 , Benjamin G Davis 3, 4 , Francisco Corzana 5 , Emilio J Cocinero 1, 2
Affiliation
|
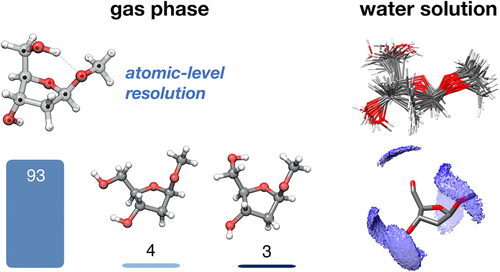
The constitution, configuration, and flexibility of the core sugars of DNA molecules alter their function in diverse roles. Conformational itineraries of the ribofuranosides (fs) have long been known to finely determine rates of processing, yet we also know that, strikingly, semifunctional DNAs containing pyranosides (ps) or other configurations can be created, suggesting sufficient but incompletely understood plasticity. The multiple conformers involved in such processes are necessarily influenced by context and environment: solvent, hosts, ligands. Notably, however, to date the unbiased, "naked" conformers have not been experimentally determined. Here, the inherent conformational biases of DNA scaffold deoxyribosides in unsolvated and solvated forms have now been defined using gas-phase microwave and solution-phase NMR spectroscopies coupled with computational analyses and exploitation of critical differences between natural-abundance isotopologues. Serial determination of precise, individual spectra for conformers of these 25 isotopologues in alpha (α-d) and beta (β-d); pyrano (p) and furano (f) methyl 2-deoxy-d-ribosides gave not only unprecedented atomic-level resolution structures of associated conformers but also their quantitative populations. Together these experiments revealed that typical 2E and 3E conformations of the sugar found in complex DNA structures are not inherently populated. Moreover, while both OH-5' and OH-3' are constrained by intramolecular hydrogen bonding in the unnatural αf scaffold, OH-3' is "born free" in the "naked" lowest lying energy conformer of natural βf. Consequently, upon solvation, unnatural αf is strikingly less perturbable (retaining 2T1 conformation in vacuo and water) than natural βf. Unnatural αp and βp ribosides also display low conformational perturbability. These first experimental data on inherent, unbiased conformers therefore suggest that it is the background of conformational flexibility of βf that may have led to its emergence out of multiple possibilities as the sugar scaffold for "life's code" and suggest a mechanism by which the resulting freedom of OH-3' (and hence accessibility as a nucleophile) in βf may drive preferential processing and complex structure formation, such as replicative propagation of DNA from 5'-to-3'.
中文翻译:

假定的DNA脚手架核糖的无偏构体的观察。
DNA分子核心糖的组成,构型和柔韧性改变了其在各种角色中的功能。早已知道核呋喃糖核苷(fs)的构象性路线可以精确确定加工速率,但我们也知道,惊人的是,可以生成含有吡喃糖苷(ps)或其他构型的半功能DNA,表明足够但不完全理解的可塑性。此类过程涉及的多个构象异构体必定会受到环境和环境的影响:溶剂,主体,配体。然而,值得注意的是,迄今为止,尚未通过实验确定无偏的“裸”构象体。这里,现已使用气相微波和液相NMR光谱结合计算分析和利用自然丰度同位素异构体之间的关键差异来定义非溶剂化和溶剂化形式的DNA支架脱氧核糖苷的固有构象偏倚。连续测定α(α-d)和β(β-d)中这25种同位素的构象的精确,单独的光谱;吡喃并(p)和呋喃并(f)甲基2-脱氧-d-核糖苷不仅提供了相关构象异构体前所未有的原子级拆分结构,还提供了其定量种群。这些实验共同表明,在复杂的DNA结构中发现的典型2E和3E糖构象并不是固有存在的。此外,虽然OH-5'和OH-3' 由于在非天然αf支架中受到分子内氢键的限制,OH-3'在天然βf的“裸露”最低能级构象异构体中“无出生”。因此,在溶剂化时,非天然αf的干扰力明显小于天然βf(在真空和水中保持2T1构象)。非天然的αp和βp核糖苷也显示出低的构象扰动性。因此,这些关于固有的,无偏见的构象异构体的第一批实验数据表明,βf构象柔性的背景可能导致其脱颖而出,成为了“生命密码”的糖支架,并提出了由此产生的自由的机制βf中的OH-3'(因此可作为亲核试剂获得)可能会推动优先加工和复杂结构的形成,
更新日期:2020-02-26
中文翻译:

假定的DNA脚手架核糖的无偏构体的观察。
DNA分子核心糖的组成,构型和柔韧性改变了其在各种角色中的功能。早已知道核呋喃糖核苷(fs)的构象性路线可以精确确定加工速率,但我们也知道,惊人的是,可以生成含有吡喃糖苷(ps)或其他构型的半功能DNA,表明足够但不完全理解的可塑性。此类过程涉及的多个构象异构体必定会受到环境和环境的影响:溶剂,主体,配体。然而,值得注意的是,迄今为止,尚未通过实验确定无偏的“裸”构象体。这里,现已使用气相微波和液相NMR光谱结合计算分析和利用自然丰度同位素异构体之间的关键差异来定义非溶剂化和溶剂化形式的DNA支架脱氧核糖苷的固有构象偏倚。连续测定α(α-d)和β(β-d)中这25种同位素的构象的精确,单独的光谱;吡喃并(p)和呋喃并(f)甲基2-脱氧-d-核糖苷不仅提供了相关构象异构体前所未有的原子级拆分结构,还提供了其定量种群。这些实验共同表明,在复杂的DNA结构中发现的典型2E和3E糖构象并不是固有存在的。此外,虽然OH-5'和OH-3' 由于在非天然αf支架中受到分子内氢键的限制,OH-3'在天然βf的“裸露”最低能级构象异构体中“无出生”。因此,在溶剂化时,非天然αf的干扰力明显小于天然βf(在真空和水中保持2T1构象)。非天然的αp和βp核糖苷也显示出低的构象扰动性。因此,这些关于固有的,无偏见的构象异构体的第一批实验数据表明,βf构象柔性的背景可能导致其脱颖而出,成为了“生命密码”的糖支架,并提出了由此产生的自由的机制βf中的OH-3'(因此可作为亲核试剂获得)可能会推动优先加工和复杂结构的形成,









































 京公网安备 11010802027423号
京公网安备 11010802027423号