当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Construction of cyclopenta[b]pyran-2-ones via chemoselective (3 + 2) cycloaddition between 2-pyrones and vinyl cyclopropanes
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020/02/19 , DOI: 10.1039/d0qo00049c Xuefeng Liang 1, 2, 3, 4 , Weijian Ye 1, 2, 3, 4 , Waygen Thor 5, 6, 7, 8 , Lantian Sun 5, 6, 7, 8 , Bo Wang 5, 6, 7, 8 , Shuzhong He 4, 9, 10, 11 , Yuen-Kit Cheng 5, 6, 7, 8 , Chi-Sing Lee 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020/02/19 , DOI: 10.1039/d0qo00049c Xuefeng Liang 1, 2, 3, 4 , Weijian Ye 1, 2, 3, 4 , Waygen Thor 5, 6, 7, 8 , Lantian Sun 5, 6, 7, 8 , Bo Wang 5, 6, 7, 8 , Shuzhong He 4, 9, 10, 11 , Yuen-Kit Cheng 5, 6, 7, 8 , Chi-Sing Lee 1, 2, 3, 4, 5
Affiliation
|
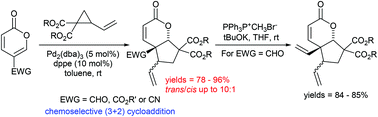
A method for synthesising cyclopenta[b]pyran-2-ones through direct (3 + 2) cycloaddition between 2-pyrones and vinyl cyclopropanes is described. In the presence of Pd2(dba)3/dppe in toluene, a series of 2-pyrones undergo chemoselective (3 + 2) cycloaddition with vinyl cyclopropanes and afford cyclopenta[b]pyran-2-ones in high yields (78–96%). The trans/cis ratio of the cycloadducts has been optimised up to 10 : 1. The proposed mechanism of this cycloaddition has been rationalised by computational studies.
中文翻译:

通过2-吡喃酮和乙烯基环丙烷之间的化学选择性(3 + 2)环加成反应构建环戊[b]吡喃-2-酮
描述了通过在2-吡喃酮和乙烯基环丙烷之间的直接(3 + 2)环加成来合成环戊[ b ]吡喃-2-酮的方法。在甲苯中存在Pd 2(dba)3 / dppe的情况下,一系列2-吡喃酮与乙烯基环丙烷进行化学选择(3 + 2)环加成反应,并以高收率获得环戊[ b ] pyran-2-ones(78-96) %)。环加合物的反式/顺式比例已优化至10:1。该环加成反应的拟议机理已通过计算研究合理化。
更新日期:2020-03-19
中文翻译:

通过2-吡喃酮和乙烯基环丙烷之间的化学选择性(3 + 2)环加成反应构建环戊[b]吡喃-2-酮
描述了通过在2-吡喃酮和乙烯基环丙烷之间的直接(3 + 2)环加成来合成环戊[ b ]吡喃-2-酮的方法。在甲苯中存在Pd 2(dba)3 / dppe的情况下,一系列2-吡喃酮与乙烯基环丙烷进行化学选择(3 + 2)环加成反应,并以高收率获得环戊[ b ] pyran-2-ones(78-96) %)。环加合物的反式/顺式比例已优化至10:1。该环加成反应的拟议机理已通过计算研究合理化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号