当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid-catalyzed pyrolytic synthesis of levoglucosan through salt-mediated ring locking
Green Chemistry ( IF 9.3 ) Pub Date : 2020/02/19 , DOI: 10.1039/c9gc03973b Li Chen 1, 2, 3, 4 , Welman C. Elias 1, 2, 3, 4 , Y. Ben Yin 1, 2, 3, 4 , Z. Conrad Zhang 5, 6, 7, 8 , Michael S. Wong 1, 2, 3, 4, 9
Green Chemistry ( IF 9.3 ) Pub Date : 2020/02/19 , DOI: 10.1039/c9gc03973b Li Chen 1, 2, 3, 4 , Welman C. Elias 1, 2, 3, 4 , Y. Ben Yin 1, 2, 3, 4 , Z. Conrad Zhang 5, 6, 7, 8 , Michael S. Wong 1, 2, 3, 4, 9
Affiliation
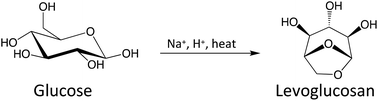
|
Selectively producing chemicals from cellulosic carbohydrate pyrolysis in large quantities is challenging, especially anhydro-monosaccharides with double-ring, triple-ring, and furan/pyran structures. Formation of these sugar derivatives greatly improves when the pyranose ring opening is inhibited during pyrolysis, which is accomplished by chemically replacing the hydroxyl group at the anomeric carbon with an alkoxy group. A simpler ring-locking approach is required for scalable chemical production, however. In this work, we demonstrate that introducing Na2SO4 and H2SO4 to glucose pyrolysis significantly increases levoglucosan (LGA) formation, from a 6% yield to as high as 40% at 350 °C. With H2SO4 as the acid catalyst, Na+ acts to inhibit the ring opening. Glucose pyrolysis with different alkali metal cations (Li+, Na+, K+, Rb+ and Cs+) gives different reaction products, which can be explained largely by an ionic electronegativity effect. Weaker electronegativity promotes the formation of a ring-opened product such as 5-hydroxymethylfurfural (HMF), and stronger electronegativity increases the formation of sequential dehydration products like levoglucosenone (LGO). Sodium has the optimum ionic electronegativity for preferential association with the ring oxygen. The Na2SO4/H2SO4 combination improved LGA yields for all carbohydrate substrates tested (up to 70%), including lignocellulose. These findings highlight the potential of using alkali metal salts to produce anhydrosugars in high yields from cellulosic carbohydrate pyrolysis.
中文翻译:

盐介导的锁环酸催化热解合成左旋葡聚糖
从纤维素碳水化合物的热解中大量选择性生产化学品具有挑战性,尤其是具有双环,三环和呋喃/吡喃结构的脱水单糖。当热解过程中抑制吡喃糖开环时,这些糖衍生物的形成大大改善,这是通过用烷氧基化学取代异头碳上的羟基来实现的。但是,对于可扩展的化学品生产,需要一种更简单的环锁方法。在这项工作中,我们证明了将Na 2 SO 4和H 2 SO 4引入葡萄糖热解显着增加了左旋葡聚糖(LGA)的形成,从6%的收率到350°C时高达40%。与H 2 SO4作为酸催化剂,Na +起到抑制开环的作用。用不同的碱金属阳离子(Li +,Na +,K +,Rb +和Cs +)进行葡萄糖热解会产生不同的反应产物,这在很大程度上可由离子电负性效应解释。较弱的电负性会促进开环产物(例如5-羟甲基糠醛(HMF))的形成,而较强的电负性会增加顺序脱水产物(如左旋葡萄糖醛酮(LGO))的形成。钠具有最佳的离子电负性,可优先与环氧缔合。Na 2 SO 4 / H 2 SO4种组合提高了所有测试的碳水化合物底物(高达70%)(包括木质纤维素)的LGA产量。这些发现凸显了使用碱金属盐从纤维素碳水化合物热解中高产量生产脱水糖的潜力。
更新日期:2020-03-24
中文翻译:

盐介导的锁环酸催化热解合成左旋葡聚糖
从纤维素碳水化合物的热解中大量选择性生产化学品具有挑战性,尤其是具有双环,三环和呋喃/吡喃结构的脱水单糖。当热解过程中抑制吡喃糖开环时,这些糖衍生物的形成大大改善,这是通过用烷氧基化学取代异头碳上的羟基来实现的。但是,对于可扩展的化学品生产,需要一种更简单的环锁方法。在这项工作中,我们证明了将Na 2 SO 4和H 2 SO 4引入葡萄糖热解显着增加了左旋葡聚糖(LGA)的形成,从6%的收率到350°C时高达40%。与H 2 SO4作为酸催化剂,Na +起到抑制开环的作用。用不同的碱金属阳离子(Li +,Na +,K +,Rb +和Cs +)进行葡萄糖热解会产生不同的反应产物,这在很大程度上可由离子电负性效应解释。较弱的电负性会促进开环产物(例如5-羟甲基糠醛(HMF))的形成,而较强的电负性会增加顺序脱水产物(如左旋葡萄糖醛酮(LGO))的形成。钠具有最佳的离子电负性,可优先与环氧缔合。Na 2 SO 4 / H 2 SO4种组合提高了所有测试的碳水化合物底物(高达70%)(包括木质纤维素)的LGA产量。这些发现凸显了使用碱金属盐从纤维素碳水化合物热解中高产量生产脱水糖的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号