当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, molecular docking, and spectral studies of new class of carbazolyl polyhydroquinoline derivatives as promising antibacterial agents with noncytotoxicity towards human mononuclear cells from peripheral blood
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-02-18 , DOI: 10.1002/jhet.3921 Karuppan Venkatapathy 1 , Chinnaiyan J. Magesh 1 , Gnanamani Lavanya 1 , Paramasivam T. Perumal 2 , Sekar Prema 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-02-18 , DOI: 10.1002/jhet.3921 Karuppan Venkatapathy 1 , Chinnaiyan J. Magesh 1 , Gnanamani Lavanya 1 , Paramasivam T. Perumal 2 , Sekar Prema 1
Affiliation
|
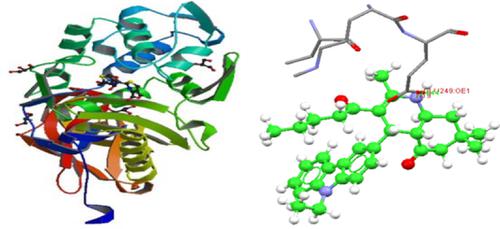
In the present study, we report the design and eco‐benign synthesis of new class of carbazolyl‐1,4‐dihydropyridine (1,4‐CDHP) and carbazolyl‐1,8‐dioxodecahydroacridine (CAD) derivatives via a three‐component coupling reaction of substituted carbazole aldehydes, ethyl acetoacetate/dimedone, and ammonium acetate under solvent‐free conditions at 112°C to 115°C. We also report an efficient one‐pot synthesis of new class of carbazolyl polyhydroquinoline (CPQ) derivatives via a four‐component coupling reaction of substituted ethyl acetoacetate, dimedone, ammonium acetate, and carbazole aldehydes in acetonitrile/water medium (3:1) at 73°C to 75°C in moderate yields. All the products were thoroughly characterized by 1H NMR, 13C NMR, Fourier transform infrared (FTIR), mass spectral, and CHN analysis. The synthesized heterocyclic compounds were evaluated for their in vitro antibacterial activity against pathogenic strains of both Gram‐negative and Gram‐positive bacteria. Minimum inhibitory concentration (MIC) of the active compounds was evaluated by macrodilution method. The CPQ derivative (8a) displayed superior antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, and Salmonella typhi with the MIC values of 16.0 to 32.0 μg/mL in comparison with the reference drug. The mechanism of antibacterial action of the CPQ derivatives was investigated via scanning electron microscope (SEM) studies. The molecular docking studies indicate that the CPQ derivative (8a) binds to the cell wall protein of E coli and P aeruginosa by formation of hydrogen bonds with amino acid residues (TYR328 and GLU249) of the bacterial cell wall protein. The 1,4‐CDHP, CAD, and CPQ derivatives were either noncytotoxic or exhibited minimal cytotoxicity towards human mononuclear cells from peripheral blood. All the products were evaluated for Lipinski rule of five (RO5) and were found to have good oral bioavailability.
中文翻译:

新型咔唑基多氢喹啉衍生物作为有前途的抗菌剂的设计,合成,分子对接和光谱研究,对外周血人单核细胞无细胞毒性
在本研究中,我们报告了通过三组分偶联作用设计的新型咔唑基-1,4-二氢吡啶(1,4-CDHP)和咔唑基-1,8-二氧十二烷基氢化hydro啶(CAD)衍生物的设计和生态友好合成取代咔唑醛,乙酰乙酸乙酯/二甲酮和乙酸铵在无溶剂条件下于112°C至115°C下反应。我们还报告了在乙腈/水介质(3:1)下,通过取代的乙酰乙酸乙酯,二甲酮,乙酸铵和咔唑醛的四组分偶联反应,可以有效地一锅合成新型咔唑基聚氢喹啉(CPQ)衍生物。 73°C至75°C,中等产量。所有的产品进行了彻底的特征在于1 H NMR,1313 C NMR,傅立叶变换红外(FTIR),质谱和CHN分析。评估了合成的杂环化合物对革兰氏阴性和革兰氏阳性细菌致病菌株的体外抗菌活性。活性化合物的最小抑菌浓度(MIC)通过宏观稀释法进行评估。CPQ衍生物(8a)对大肠杆菌,铜绿假单胞菌和伤寒沙门氏菌表现出优异的抗菌活性。与参考药物相比的MIC值为16.0至32.0μg/ mL。通过扫描电子显微镜(SEM)研究了CPQ衍生物的抗菌作用机理。分子对接研究表明,CPQ衍生物(8a)通过与细菌细胞壁蛋白的氨基酸残基(TYR328和GLU249)形成氢键与大肠杆菌和铜绿假单胞菌的细胞壁蛋白结合。1,4-CDHP,CAD和CPQ衍生物无细胞毒性,或对外周血中人单核细胞的细胞毒性最小。对所有产品进行了Lipinski 5规则(RO5)评估,发现它们具有良好的口服生物利用度。
更新日期:2020-02-19
中文翻译:

新型咔唑基多氢喹啉衍生物作为有前途的抗菌剂的设计,合成,分子对接和光谱研究,对外周血人单核细胞无细胞毒性
在本研究中,我们报告了通过三组分偶联作用设计的新型咔唑基-1,4-二氢吡啶(1,4-CDHP)和咔唑基-1,8-二氧十二烷基氢化hydro啶(CAD)衍生物的设计和生态友好合成取代咔唑醛,乙酰乙酸乙酯/二甲酮和乙酸铵在无溶剂条件下于112°C至115°C下反应。我们还报告了在乙腈/水介质(3:1)下,通过取代的乙酰乙酸乙酯,二甲酮,乙酸铵和咔唑醛的四组分偶联反应,可以有效地一锅合成新型咔唑基聚氢喹啉(CPQ)衍生物。 73°C至75°C,中等产量。所有的产品进行了彻底的特征在于1 H NMR,1313 C NMR,傅立叶变换红外(FTIR),质谱和CHN分析。评估了合成的杂环化合物对革兰氏阴性和革兰氏阳性细菌致病菌株的体外抗菌活性。活性化合物的最小抑菌浓度(MIC)通过宏观稀释法进行评估。CPQ衍生物(8a)对大肠杆菌,铜绿假单胞菌和伤寒沙门氏菌表现出优异的抗菌活性。与参考药物相比的MIC值为16.0至32.0μg/ mL。通过扫描电子显微镜(SEM)研究了CPQ衍生物的抗菌作用机理。分子对接研究表明,CPQ衍生物(8a)通过与细菌细胞壁蛋白的氨基酸残基(TYR328和GLU249)形成氢键与大肠杆菌和铜绿假单胞菌的细胞壁蛋白结合。1,4-CDHP,CAD和CPQ衍生物无细胞毒性,或对外周血中人单核细胞的细胞毒性最小。对所有产品进行了Lipinski 5规则(RO5)评估,发现它们具有良好的口服生物利用度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号