当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Ortho-Olefination of Sterically Demanding Benzamides: Application to Asymmetric Synthesis of Axially Chiral Benzamides.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-02-19 , DOI: 10.1002/chem.202000797 Ryo Yoshimura 1 , Ken Tanaka 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-02-19 , DOI: 10.1002/chem.202000797 Ryo Yoshimura 1 , Ken Tanaka 1
Affiliation
|
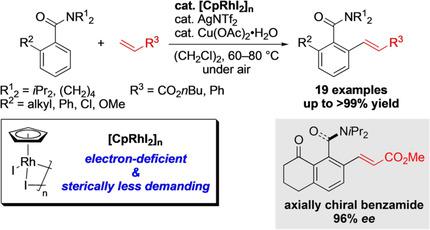
It has been established that an unsubstituted cyclopentadienyl rhodium(III) (CpRh III ) complex is a highly active catalyst for the aerobic oxidative ortho C-H bond olefination of sterically demanding ortho -substituted benzamides with alkenes. This catalysis was successfully applied to the diastereoselective synthesis of axially chiral N , N- dialkylbenzamides. The combination of the ruthenium(II)-catalyzed enantioselective hydrogenation and the CpRh III -catalyzed diastereoselective ortho C-H bond olefination enabled the asymmetric synthesis of axially chiral N , N- dialkylbenzamide derivatives with high ee values.
中文翻译:

铑催化的对立体要求高的苯甲酰胺的邻位加成:在轴对称手性苯甲酰胺的不对称合成中的应用。
已经确定的是,未取代的环戊二烯基铑(III)(CpRh III)配合物是用于空间上要求的邻位取代的苯甲酰胺与烯烃的需氧氧化邻位CH键烯烃的高活性催化剂。该催化成功地用于轴向手性N,N-二烷基苯甲酰胺的非对映选择性合成。钌(II)催化的对映选择性氢化和CpRh III催化的非对映选择性邻位CH键烯烃聚合可实现不对称合成具有高ee值的轴向手性N,N-二烷基苯甲酰胺衍生物。
更新日期:2020-03-24
中文翻译:

铑催化的对立体要求高的苯甲酰胺的邻位加成:在轴对称手性苯甲酰胺的不对称合成中的应用。
已经确定的是,未取代的环戊二烯基铑(III)(CpRh III)配合物是用于空间上要求的邻位取代的苯甲酰胺与烯烃的需氧氧化邻位CH键烯烃的高活性催化剂。该催化成功地用于轴向手性N,N-二烷基苯甲酰胺的非对映选择性合成。钌(II)催化的对映选择性氢化和CpRh III催化的非对映选择性邻位CH键烯烃聚合可实现不对称合成具有高ee值的轴向手性N,N-二烷基苯甲酰胺衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号