当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular Proton Transfer Controls Protein Structural Changes in Phytochrome.
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-28 , DOI: 10.1021/acs.biochem.0c00053 Anastasia Kraskov 1 , Anh Duc Nguyen 1 , Jan Goerling 1 , David Buhrke 1 , Francisco Velazquez Escobar 1 , Maria Fernandez Lopez 1 , Norbert Michael 1 , Luisa Sauthof 2 , Andrea Schmidt 2 , Patrick Piwowarski 3 , Yang Yang 4 , Till Stensitzki 4 , Suliman Adam 5 , Franz Bartl 3 , Igor Schapiro 5 , Karsten Heyne 4 , Friedrich Siebert 6 , Patrick Scheerer 2 , Maria Andrea Mroginski 1 , Peter Hildebrandt 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-28 , DOI: 10.1021/acs.biochem.0c00053 Anastasia Kraskov 1 , Anh Duc Nguyen 1 , Jan Goerling 1 , David Buhrke 1 , Francisco Velazquez Escobar 1 , Maria Fernandez Lopez 1 , Norbert Michael 1 , Luisa Sauthof 2 , Andrea Schmidt 2 , Patrick Piwowarski 3 , Yang Yang 4 , Till Stensitzki 4 , Suliman Adam 5 , Franz Bartl 3 , Igor Schapiro 5 , Karsten Heyne 4 , Friedrich Siebert 6 , Patrick Scheerer 2 , Maria Andrea Mroginski 1 , Peter Hildebrandt 1
Affiliation
|
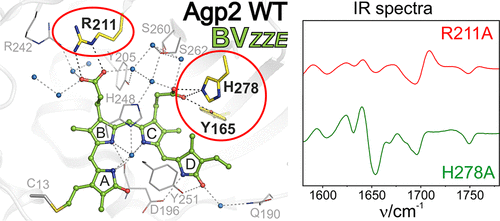
Phytochromes are biological photoswitches that interconvert between two parent states (Pr and Pfr). The transformation is initiated by photoisomerization of the tetrapyrrole chromophore, followed by a sequence of chromophore and protein structural changes. In the last step, a phytochrome-specific peptide segment (tongue) undergoes a secondary structure change, which in prokaryotic phytochromes is associated with the (de)activation of the output module. The focus of this work is the Pfr-to-Pr photoconversion of the bathy bacteriophytochrome Agp2 in which Pfr is the thermodynamically stable state. Using spectroscopic techniques, we studied the structural and functional consequences of substituting Arg211, Tyr165, His278, and Phe192 close to the biliverdin (BV) chromophore. In Pfr, substitutions of these residues do not affect the BV structure. The characteristic Pfr properties of bathy phytochromes, including the protonated propionic side chain of ring C (propC) of BV, are preserved. However, replacing Arg211 or Tyr165 blocks the photoconversion in the Meta-F state, prior to the secondary structure transition of the tongue and without deprotonation of propC. The Meta-F state of these variants displays low photochemical activity, but electronic excitation causes ultrafast alterations of the hydrogen bond network surrounding the chromophore. In all variants studied here, thermal back conversion from the photoproducts to Pfr is decelerated but substitution of His278 or Phe192 is not critical for the Pfr-to-Pr photoconversion. These variants do not impair deprotonation of propC or the α-helix/β-sheet transformation of the tongue during the Meta-F-to-Pr decay. Thus, we conclude that propC deprotonation is essential for restructuring of the tongue.
中文翻译:

分子内质子转移控制植物色素的蛋白质结构变化。
植物色素是在两个母体状态(Pr和Pfr)之间相互转换的生物光开关。转化是通过四吡咯发色团的光异构化开始的,随后是发色团和蛋白质结构变化的序列。在最后一步中,植物色素特定的肽段(舌)经历了二级结构变化,这在原核植物色素中与输出模块的(去激活)相关。这项工作的重点是浴状细菌植物色素Agp2的Pfr到Pr的光转化,其中Pfr是热力学稳定状态。使用光谱技术,我们研究了取代Biliverdin(BV)发色团的Arg211,Tyr165,His278和Phe192的结构和功能后果。在Pfr中,这些残基的取代不影响BV结构。保留了水生植物色素的特征性Pfr特性,包括BV环C(propC)的质子化丙酸侧链。然而,在舌头的二级结构转变之前,替换Arg211或Tyr165会阻止Meta-F状态下的光转化,并且不会使propC脱质子。这些变体的Meta-F状态显示出较低的光化学活性,但是电子激发导致发色团周围的氢键网络发生超快速变化。在本文研究的所有变体中,从光产物到Pfr的热逆转换都被减速,但是His278或Phe192的取代对于Pfr到Pr的光转换不是关键。这些变体不会在Meta-F-to-Pr衰变期间损害propC的去质子化或舌头的α-螺旋/β-折叠转变。从而,
更新日期:2020-02-28
中文翻译:

分子内质子转移控制植物色素的蛋白质结构变化。
植物色素是在两个母体状态(Pr和Pfr)之间相互转换的生物光开关。转化是通过四吡咯发色团的光异构化开始的,随后是发色团和蛋白质结构变化的序列。在最后一步中,植物色素特定的肽段(舌)经历了二级结构变化,这在原核植物色素中与输出模块的(去激活)相关。这项工作的重点是浴状细菌植物色素Agp2的Pfr到Pr的光转化,其中Pfr是热力学稳定状态。使用光谱技术,我们研究了取代Biliverdin(BV)发色团的Arg211,Tyr165,His278和Phe192的结构和功能后果。在Pfr中,这些残基的取代不影响BV结构。保留了水生植物色素的特征性Pfr特性,包括BV环C(propC)的质子化丙酸侧链。然而,在舌头的二级结构转变之前,替换Arg211或Tyr165会阻止Meta-F状态下的光转化,并且不会使propC脱质子。这些变体的Meta-F状态显示出较低的光化学活性,但是电子激发导致发色团周围的氢键网络发生超快速变化。在本文研究的所有变体中,从光产物到Pfr的热逆转换都被减速,但是His278或Phe192的取代对于Pfr到Pr的光转换不是关键。这些变体不会在Meta-F-to-Pr衰变期间损害propC的去质子化或舌头的α-螺旋/β-折叠转变。从而,









































 京公网安备 11010802027423号
京公网安备 11010802027423号