当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Natural Products with Bridged Bicyclo[m.n.1] Ring Systems via Type II [5 + 2] Cycloaddition.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-02-18 , DOI: 10.1021/acs.accounts.9b00640 Long Min 1 , Xin Liu 1 , Chuang-Chuang Li 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-02-18 , DOI: 10.1021/acs.accounts.9b00640 Long Min 1 , Xin Liu 1 , Chuang-Chuang Li 1
Affiliation
|
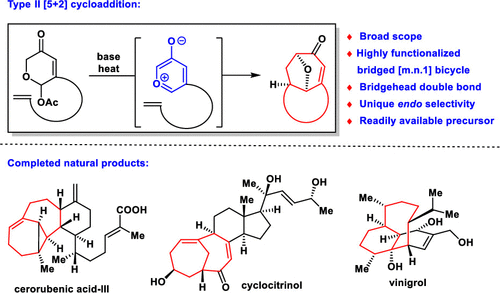
Natural products containing bridged ring systems are widely identified and show significant biological activity. The development of efficient synthesis reactions and strategies to construct bridged ring systems is a long-standing but very significant challenge in organic chemistry. In 2014, our group developed a unique type II [5 + 2] cycloaddition reaction that provides a facile and direct methodology for constructing highly functionalized bridged bicyclo[4.3.1], bicyclo[4.4.1], bicyclo[5.4.1], bicyclo[6.4.1], and other bicyclo[m.n.1] systems containing a strained bridgehead double bond. In this Account, we summarize the methodology development and report the results of application of our unique strategy for the total synthesis of several natural products with bridged ring systems (i.e., cyclocitrinol, cerorubenic acid-III, and vinigrol) during the past 5 years in our laboratory. In the first part, we introduce the logic behind the design and discovery of type II [5 + 2] cycloadditions. The substrates can be easily synthesized by a modular approach, followed by base-promoted group elimination under heat to form an oxidopyrylium ylide, which can undergo cycloaddition under relatively mild conditions with a variety of double bonds to generate bridged bicyclo[m.n.1] frameworks in high yield. The diastereocontrol and unique endo selectivity of this methodology are favorable for further application to the synthesis of complex natural products. In the second part, we highlight our endeavors in the total synthesis of several different types of molecules bearing bridged ring systems using our methodology. The bridged bicyclo[4.4.1] system is the core structure of two different types of natural products, cyclocitrinol and cerorubenic acid-III, that can be efficiently constructed by type II [5 + 2] cycloadditions. The development of suitable strategies and methods for site-selective cleavage of the C-O bond of the oxa-[3.2.1] ring system in the products of type II [5 + 2] cycloadditions is also discussed and highlighted during the syntheses. Moreover, the bridged bicyclo[5.3.1] system is the core structure of vinigrol, which can be constructed through a novel ring contraction sequence of the bicyclo[5.4.1] system formed by a type II [5 + 2] cycloaddition. By combining with a ring contraction cascade, we believe that type II [5 + 2] cycloadditions have the potential to be used as a unified approach to constructing natural products containing bridged bicyclo[m.n.1] frameworks.
中文翻译:

通过II型[5 + 2]环加成与桥联双环[mn1]环系统合成天然产物。
包含桥环系统的天然产物已被广泛鉴定并显示出显着的生物学活性。在有机化学中,开发有效的合成反应和构建桥环系统的策略是一个长期但非常重要的挑战。2014年,我们小组开发了独特的II型[5 + 2]环加成反应,为构建高度官能化的桥连双环[4.3.1],双环[4.4.1],双环[5.4.1], bicyclo [6.4.1]和其他包含应变桥头双键的bicyclo [mn1]系统。在此帐户中,我们总结了方法学的发展,并报告了我们独特的策略用于几种具有桥环系统的天然产物(即环西三醇,cerorubenic acid-III,和vinigrol)在过去五年中在我们的实验室中。在第一部分中,我们介绍了II型[5 + 2]环加成的设计和发现背后的逻辑。可以通过模块化方法轻松合成底物,然后在加热下通过碱促进基团消除形成氧化吡啶鎓叶立德,该氧化吡啶鎓叶立德可以在相对温和的条件下进行环加成,并带有各种双键,从而在较高的温度下生成桥联的双环[mn1]骨架让。这种方法的非对映控制和独特的内在选择性有利于进一步应用于复杂天然产物的合成。在第二部分中,我们着重介绍了使用我们的方法对几种带有桥环系统的分子进行全合成的努力。桥联双环[4.4。1]系统是两种不同类型天然产物的核心结构,环环三醇和铜酸III,可以通过II型[5 + 2]环加成反应有效地构建。在合成过程中,还讨论并强调了在II型[5 + 2]环加成产物中对oxa- [3.2.1]环系统的CO键进行位点选择性裂解的合适策略和方法的开发。而且,桥联的双环[5.3.1]系统是长春瑞醇的核心结构,可以通过II型[5 + 2]环加成反应形成的双环[5.4.1]系统的新型环收缩序列来构建。通过与环收缩级联反应相结合,我们相信II型[5 + 2]环加成物有可能被用作构建包含桥接双环[mn1]骨架的天然产物的统一方法。
更新日期:2020-02-19
中文翻译:

通过II型[5 + 2]环加成与桥联双环[mn1]环系统合成天然产物。
包含桥环系统的天然产物已被广泛鉴定并显示出显着的生物学活性。在有机化学中,开发有效的合成反应和构建桥环系统的策略是一个长期但非常重要的挑战。2014年,我们小组开发了独特的II型[5 + 2]环加成反应,为构建高度官能化的桥连双环[4.3.1],双环[4.4.1],双环[5.4.1], bicyclo [6.4.1]和其他包含应变桥头双键的bicyclo [mn1]系统。在此帐户中,我们总结了方法学的发展,并报告了我们独特的策略用于几种具有桥环系统的天然产物(即环西三醇,cerorubenic acid-III,和vinigrol)在过去五年中在我们的实验室中。在第一部分中,我们介绍了II型[5 + 2]环加成的设计和发现背后的逻辑。可以通过模块化方法轻松合成底物,然后在加热下通过碱促进基团消除形成氧化吡啶鎓叶立德,该氧化吡啶鎓叶立德可以在相对温和的条件下进行环加成,并带有各种双键,从而在较高的温度下生成桥联的双环[mn1]骨架让。这种方法的非对映控制和独特的内在选择性有利于进一步应用于复杂天然产物的合成。在第二部分中,我们着重介绍了使用我们的方法对几种带有桥环系统的分子进行全合成的努力。桥联双环[4.4。1]系统是两种不同类型天然产物的核心结构,环环三醇和铜酸III,可以通过II型[5 + 2]环加成反应有效地构建。在合成过程中,还讨论并强调了在II型[5 + 2]环加成产物中对oxa- [3.2.1]环系统的CO键进行位点选择性裂解的合适策略和方法的开发。而且,桥联的双环[5.3.1]系统是长春瑞醇的核心结构,可以通过II型[5 + 2]环加成反应形成的双环[5.4.1]系统的新型环收缩序列来构建。通过与环收缩级联反应相结合,我们相信II型[5 + 2]环加成物有可能被用作构建包含桥接双环[mn1]骨架的天然产物的统一方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号