当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Properties of α-Synuclein Secondary Nuclei Are Dominated by the Solution Conditions Rather than the Seed Fibril Strain.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-02-28 , DOI: 10.1021/acschemneuro.9b00594 Alessia Peduzzo 1 , Sara Linse 2 , Alexander K Buell 1, 3
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-02-28 , DOI: 10.1021/acschemneuro.9b00594 Alessia Peduzzo 1 , Sara Linse 2 , Alexander K Buell 1, 3
Affiliation
|
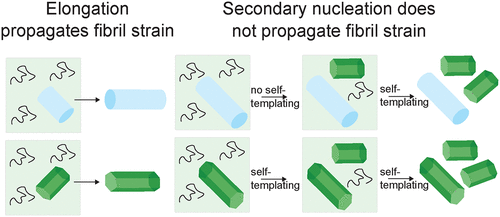
Amyloid fibrils of α-synuclein (α-syn) are a component of Lewy bodies, the characteristic hallmark of Parkinson's disease. Amyloid fibrils arise through primary nucleation from monomers, which in the case of α-syn is often heterogeneous, followed by the growth of the nuclei by monomer addition. Secondary nucleation corresponds to the formation of new fibrils facilitated by pre-existing fibrils. While it is well-established that the newly added monomer in fibril elongation adopts the conformation of the monomers in the seed ("templating"), it is unclear whether fibrils formed through secondary nucleation of monomers on the surface of seed fibrils copy the structure of the "parent" fibril. Here we show by biochemical and microscopical methods that the secondary nucleation of α-syn, enabled at mildly acidic pH, leads to fibrils that structurally resemble more closely those formed de novo under the same conditions, rather than the seeds if these are formed under different solution conditions. This result has important implications for the mechanistic understanding of the secondary nucleation of amyloid fibrils and its role in the propagation of aggregate pathology in protein misfolding diseases.
中文翻译:

α-突触核蛋白二级核的性质由溶液条件决定,而不是由种子原纤维菌株决定。
α-突触核蛋白(α-syn)的淀粉样原纤维是路易体的组成部分,路易体是帕金森氏病的特征性标志。淀粉样蛋白原纤维通过单体的初级成核而产生,在α-syn的情况下,淀粉样原纤维通常是异质的,随后通过单体添加使核生长。二次成核对应于通过预先存在的原纤维促进新的原纤维的形成。众所周知,原纤维伸长中新加入的单体采用了种子中单体的构象(“模板化”),但尚不清楚通过种子原纤维表面上的单体二次成核形成的原纤维是否复制了种子的结构。 “父”原纤维。在这里,我们通过生化和显微镜方法表明,在中等酸性pH下,α-syn的二次成核,导致原纤维在结构上更像是在相同条件下从头形成的原纤维,而不是种子,如果它们是在不同溶液条件下形成的。该结果对于淀粉样蛋白原纤维继发成核的机理理解及其在蛋白质错误折叠疾病中聚集病理学传播中的作用具有重要意义。
更新日期:2020-03-02
中文翻译:

α-突触核蛋白二级核的性质由溶液条件决定,而不是由种子原纤维菌株决定。
α-突触核蛋白(α-syn)的淀粉样原纤维是路易体的组成部分,路易体是帕金森氏病的特征性标志。淀粉样蛋白原纤维通过单体的初级成核而产生,在α-syn的情况下,淀粉样原纤维通常是异质的,随后通过单体添加使核生长。二次成核对应于通过预先存在的原纤维促进新的原纤维的形成。众所周知,原纤维伸长中新加入的单体采用了种子中单体的构象(“模板化”),但尚不清楚通过种子原纤维表面上的单体二次成核形成的原纤维是否复制了种子的结构。 “父”原纤维。在这里,我们通过生化和显微镜方法表明,在中等酸性pH下,α-syn的二次成核,导致原纤维在结构上更像是在相同条件下从头形成的原纤维,而不是种子,如果它们是在不同溶液条件下形成的。该结果对于淀粉样蛋白原纤维继发成核的机理理解及其在蛋白质错误折叠疾病中聚集病理学传播中的作用具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号