当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Scalable Process for the PPAR-α Agonist GW641597X Incorporating Baeyer–Villiger Chemistry and Retrospective ICH M7 Assessment
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-03-05 , DOI: 10.1021/acs.oprd.9b00385 Thierry Boyer 1 , Bernie M. Choudary 2 , Andrew J. Edwards 3 , Erika Etridge 2 , Steve Etridge 2 , Amanda Giddings 4 , Jim Harvey 4 , Neil S. Hodnett 2, 3 , Sophie Druot-Houllemare 2 , John H. Leahy 3 , Kathryn Payne 2 , Robert Reid 2, 3 , Andrew D. Roberts 3 , Mike Sasse 2 , Alec Simpson 3 , Steve Smith 2 , Neil G. Stevenson 3 , Paul Stonestreet 2 , Michael William Urquhart 2, 3 , Angela White 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-03-05 , DOI: 10.1021/acs.oprd.9b00385 Thierry Boyer 1 , Bernie M. Choudary 2 , Andrew J. Edwards 3 , Erika Etridge 2 , Steve Etridge 2 , Amanda Giddings 4 , Jim Harvey 4 , Neil S. Hodnett 2, 3 , Sophie Druot-Houllemare 2 , John H. Leahy 3 , Kathryn Payne 2 , Robert Reid 2, 3 , Andrew D. Roberts 3 , Mike Sasse 2 , Alec Simpson 3 , Steve Smith 2 , Neil G. Stevenson 3 , Paul Stonestreet 2 , Michael William Urquhart 2, 3 , Angela White 4
Affiliation
|
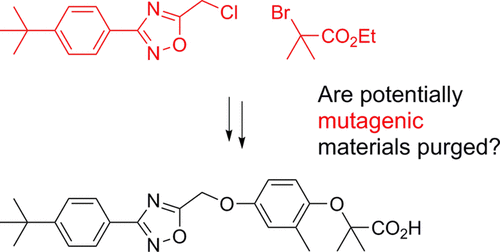
The development of the synthetic process to the PPAR-α receptor antagonist 5-((4-(tert-butoxy)-3-methylphenoxy)methyl)-3-(4-(tert-butyl)phenyl)-1,2,4-oxadiazole (GW641597X) 1 is described. The discussion ranges from the initial supply route, used to deliver early batches for preliminary safety studies to enable dosing in man, to an efficient manufacturing route, which delivered 35 kg of drug substance following on from a pilot plant campaign. The process includes a key oxidative Baeyer–Villiger reaction, where process development identified sodium perborate in acetic acid as a safer alternative to m-chloroperoxybenzoic acid that was used in the initial supply route. Described within is a discussion of impurities, how they are formed, and the process modifications incorporated to either reduce or remove them. There is also a discussion of potential mutagenic impurities within the synthetic process and a retrospective evaluation using ICH M7 control options. Finally, an alternative route to GW641597X is described, which offers the advantage that all intermediates are crystalline facilitating material handling, offering purification opportunities through recrystallization if required, and potentially providing greater controls for the process. This new route was also retrospectively assessed using ICH M7 option controls and highlighted that ICH M7 option 4 controls can be implemented even with excess mutagenic reagents being introduced near to the drug substance as long as the science for its purging is well understood.
中文翻译:

结合Baeyer-Villiger化学和回顾性ICH M7评估的PPAR-α激动剂GW641597X的可扩展方法的开发
PPAR-α受体拮抗剂5-(((4-(叔丁氧基)-3-甲基苯氧基)甲基)-3-(4-(叔丁基)苯基)-1,2,4的合成方法的发展描述了-恶二唑(GW641597X)1。讨论的范围从最初的供应途径开始,用于为早期安全研究提供早期批次以进行人为剂量,再到有效的制造途径,在试点工厂活动之后,该途径提供了35千克原料药。该过程包括键氧化拜尔-维利格反应,其中工艺开发鉴定过硼酸钠的乙酸作为一种更安全的替代米最初的供应途径中使用的-氯过氧苯甲酸。其中讨论了杂质,杂质的形成方式以及为减少或去除杂质而进行的工艺改进。还讨论了合成过程中潜在的诱变杂质,并使用ICH M7控制选项进行了回顾性评估。最后,描述了通往GW641597X的替代路线,该路线的优点是所有中间体均为晶体,有利于材料处理,如果需要,可通过重结晶提供纯化机会,并可能为工艺提供更好的控制。
更新日期:2020-03-05
中文翻译:

结合Baeyer-Villiger化学和回顾性ICH M7评估的PPAR-α激动剂GW641597X的可扩展方法的开发
PPAR-α受体拮抗剂5-(((4-(叔丁氧基)-3-甲基苯氧基)甲基)-3-(4-(叔丁基)苯基)-1,2,4的合成方法的发展描述了-恶二唑(GW641597X)1。讨论的范围从最初的供应途径开始,用于为早期安全研究提供早期批次以进行人为剂量,再到有效的制造途径,在试点工厂活动之后,该途径提供了35千克原料药。该过程包括键氧化拜尔-维利格反应,其中工艺开发鉴定过硼酸钠的乙酸作为一种更安全的替代米最初的供应途径中使用的-氯过氧苯甲酸。其中讨论了杂质,杂质的形成方式以及为减少或去除杂质而进行的工艺改进。还讨论了合成过程中潜在的诱变杂质,并使用ICH M7控制选项进行了回顾性评估。最后,描述了通往GW641597X的替代路线,该路线的优点是所有中间体均为晶体,有利于材料处理,如果需要,可通过重结晶提供纯化机会,并可能为工艺提供更好的控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号