当前位置:
X-MOL 学术
›
Oncogenesis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chromatin remodeling protein HELLS is critical for retinoblastoma tumor initiation and progression.
Oncogenesis ( IF 5.9 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41389-020-0210-7 Loredana Zocchi 1 , Aditi Mehta 2, 3 , Stephanie C Wu 4 , Jie Wu 5, 6 , Yijun Gu 1 , Jingtian Wang 7 , Susie Suh 8 , Robert C Spitale 1, 7 , Claudia A Benavente 1, 4, 6
Oncogenesis ( IF 5.9 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41389-020-0210-7 Loredana Zocchi 1 , Aditi Mehta 2, 3 , Stephanie C Wu 4 , Jie Wu 5, 6 , Yijun Gu 1 , Jingtian Wang 7 , Susie Suh 8 , Robert C Spitale 1, 7 , Claudia A Benavente 1, 4, 6
Affiliation
|
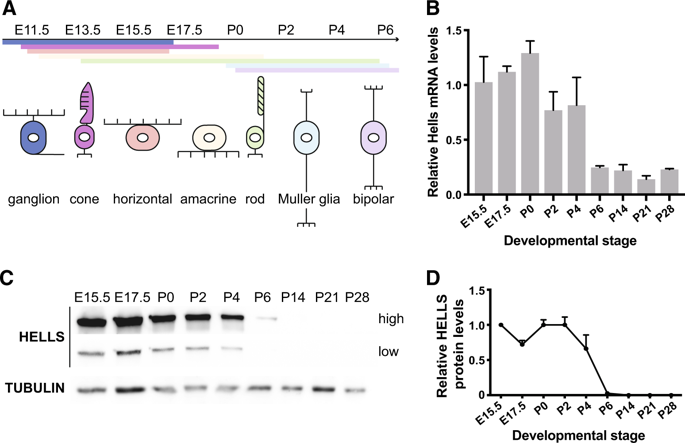
Retinoblastoma is an aggressive childhood cancer of the developing retina that initiates by biallelic RB1 gene inactivation. Tumor progression in retinoblastoma is driven by epigenetics, as retinoblastoma genomes are stable, but the mechanism(s) that drive these epigenetic changes remain unknown. Lymphoid-specific helicase (HELLS) protein is an epigenetic modifier directly regulated by the RB/E2F pathway. In this study, we used novel genetically engineered mouse models to investigate the role of HELLS during retinal development and tumorigenesis. Our results indicate that Hells-null retinal progenitor cells divide, undergo cell-fate specification, and give rise to fully laminated retinae with minor bipolar cells defects, but normal retinal function. Despite the apparent nonessential role of HELLS in retinal development, failure to transcriptionally repress Hells during retinal terminal differentiation due to retinoblastoma (RB) family loss significantly contributes to retinal tumorigenesis. Loss of HELLS drastically reduced ectopic division of differentiating cells in Rb1/p107-null retinae, significantly decreased the incidence of retinoblastoma, delayed tumor progression, and increased overall survival. Despite its role in heterochromatin formation, we found no evidence that Hells loss directly affected chromatin accessibility in the retina but functioned as transcriptional co-activator of E2F3, decreasing expression of cell cycle genes. We propose that HELLS is a critical downstream mediator of E2F-dependent ectopic proliferation in RB-null retinae. Together with the nontoxic effect of HELLS loss in the developing retina, our results suggest that HELLS and its downstream pathways could serve as potential therapeutic targets for retinoblastoma.
中文翻译:

染色质重塑蛋白HELLS对于视网膜母细胞瘤肿瘤的发生和发展至关重要。
视网膜母细胞瘤是一种发展中的视网膜的儿童期恶性肿瘤,其由双等位基因RB1基因失活引发。视网膜母细胞瘤的肿瘤进展是由表观遗传学驱动的,因为视网膜母细胞瘤的基因组是稳定的,但是驱动这些表观遗传学改变的机制仍然未知。淋巴特异性解旋酶(HELLS)蛋白是一种由RB / E2F途径直接调控的表观遗传修饰剂。在这项研究中,我们使用新型的基因工程小鼠模型来研究HELLS在视网膜发育和肿瘤发生过程中的作用。我们的结果表明,Hells-null视网膜祖细胞分裂,经历细胞命运的规范,并产生完全叠层的视网膜,并伴有轻微的双极细胞缺陷,但视网膜功能正常。尽管HELLS在视网膜发育中具有明显的非必需作用,由于视网膜母细胞瘤(RB)家族丧失,在视网膜终末分化过程中未能转录抑制地狱,这是导致视网膜肿瘤发生的重要原因。HELLS的丧失极大地减少了Rb1 / p107-null视网膜中分化细胞的异位分裂,显着降低了视网膜母细胞瘤的发生率,延迟了肿瘤的进展并提高了总生存期。尽管其在异染色质形成中的作用,我们发现没有证据表明地狱的损失直接影响视网膜中染色质的可及性,但起E2F3的转录共激活因子的作用,降低了细胞周期基因的表达。我们建议HELLS是RB空视网膜中E2F依赖性异位增殖的关键下游介质。再加上HELLS在发育中的视网膜中的无毒作用,
更新日期:2020-02-18
中文翻译:

染色质重塑蛋白HELLS对于视网膜母细胞瘤肿瘤的发生和发展至关重要。
视网膜母细胞瘤是一种发展中的视网膜的儿童期恶性肿瘤,其由双等位基因RB1基因失活引发。视网膜母细胞瘤的肿瘤进展是由表观遗传学驱动的,因为视网膜母细胞瘤的基因组是稳定的,但是驱动这些表观遗传学改变的机制仍然未知。淋巴特异性解旋酶(HELLS)蛋白是一种由RB / E2F途径直接调控的表观遗传修饰剂。在这项研究中,我们使用新型的基因工程小鼠模型来研究HELLS在视网膜发育和肿瘤发生过程中的作用。我们的结果表明,Hells-null视网膜祖细胞分裂,经历细胞命运的规范,并产生完全叠层的视网膜,并伴有轻微的双极细胞缺陷,但视网膜功能正常。尽管HELLS在视网膜发育中具有明显的非必需作用,由于视网膜母细胞瘤(RB)家族丧失,在视网膜终末分化过程中未能转录抑制地狱,这是导致视网膜肿瘤发生的重要原因。HELLS的丧失极大地减少了Rb1 / p107-null视网膜中分化细胞的异位分裂,显着降低了视网膜母细胞瘤的发生率,延迟了肿瘤的进展并提高了总生存期。尽管其在异染色质形成中的作用,我们发现没有证据表明地狱的损失直接影响视网膜中染色质的可及性,但起E2F3的转录共激活因子的作用,降低了细胞周期基因的表达。我们建议HELLS是RB空视网膜中E2F依赖性异位增殖的关键下游介质。再加上HELLS在发育中的视网膜中的无毒作用,











































 京公网安备 11010802027423号
京公网安备 11010802027423号