当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ni-Catalyzed reductive amination of phenols with ammonia or amines into cyclohexylamines
Green Chemistry ( IF 9.8 ) Pub Date : 2020/02/18 , DOI: 10.1039/c9gc02625h Thomas Cuypers 1, 2, 3, 4, 5 , Thomas Morias 1, 2, 3, 4, 5 , Simon Windels 1, 2, 3, 4, 5 , Carlos Marquez 1, 2, 3, 4, 5 , Cédric Van Goethem 1, 2, 3, 4, 5 , Ivo Vankelecom 1, 2, 3, 4, 5 , Dirk E. De Vos 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2020/02/18 , DOI: 10.1039/c9gc02625h Thomas Cuypers 1, 2, 3, 4, 5 , Thomas Morias 1, 2, 3, 4, 5 , Simon Windels 1, 2, 3, 4, 5 , Carlos Marquez 1, 2, 3, 4, 5 , Cédric Van Goethem 1, 2, 3, 4, 5 , Ivo Vankelecom 1, 2, 3, 4, 5 , Dirk E. De Vos 1, 2, 3, 4, 5
Affiliation
|
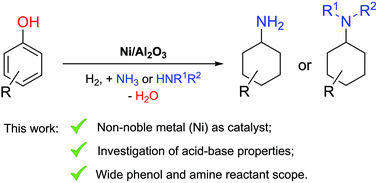
Phenol and its derivatives, which naturally occur in lignocellulose, can be considered as a renewable feedstock not only for aromatic, but also for alicyclic compounds, such as primary and N-substituted cyclohexylamines. So far, the latter are mostly produced from non-renewable starting materials like benzene via problematic nitration/reduction or cross-coupling routes. Herein, an efficient reductive amination of phenol with ammonia or amines is demonstrated, for the first time without the need for rare and expensive noble metals and without using any additives. Various supported Ni catalysts were screened and we elucidated the influence of the key parameters, including the acid–base properties of the supporting material. Acquired knowledge was then applied to different phenol–ammonia/amine combinations, resulting in the synthesis of various primary, secondary and tertiary cyclohexylamines in fair to very high yields.
中文翻译:

Ni催化的苯酚与氨或胺的还原胺化反应成环己胺
天然存在于木质纤维素中的苯酚及其衍生物不仅可以用于芳族化合物,还可以用于脂环族化合物(例如伯和N-取代的环己胺)作为可再生原料。到目前为止,后者主要由不可再生的原材料(例如苯)经硝化/还原或交叉偶联途径有问题。在本文中,首次证明了苯酚与氨或胺的有效还原胺化,而无需稀有和昂贵的贵金属且无需使用任何添加剂。筛选了各种负载型Ni催化剂,我们阐明了关键参数的影响,包括载体材料的酸碱性质。然后将获得的知识应用于不同的苯酚-氨/胺组合,从而以相当高的收率合成了各种伯,仲和叔环己胺。
更新日期:2020-03-24
中文翻译:

Ni催化的苯酚与氨或胺的还原胺化反应成环己胺
天然存在于木质纤维素中的苯酚及其衍生物不仅可以用于芳族化合物,还可以用于脂环族化合物(例如伯和N-取代的环己胺)作为可再生原料。到目前为止,后者主要由不可再生的原材料(例如苯)经硝化/还原或交叉偶联途径有问题。在本文中,首次证明了苯酚与氨或胺的有效还原胺化,而无需稀有和昂贵的贵金属且无需使用任何添加剂。筛选了各种负载型Ni催化剂,我们阐明了关键参数的影响,包括载体材料的酸碱性质。然后将获得的知识应用于不同的苯酚-氨/胺组合,从而以相当高的收率合成了各种伯,仲和叔环己胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号